可生物降解的酸响应纳米载体,通过抑制尿素酶加强抗生素治疗抗药性幽门螺旋杆菌的效果
IF 18.5
1区 材料科学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
本文章由计算机程序翻译,如有差异,请以英文原文为准。
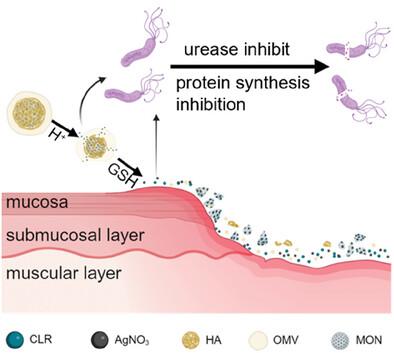
Biodegradable Acid-Responsive Nanocarrier for Enhanced Antibiotic Therapy Against Drug-Resistant Helicobacter Pylori via Urease Inhibition
Metal ion-based inhibition of urease activity is a promising strategy for treating Helicobacter pylori (H. pylori) infections. However, the challenges of safe delivery and reducing cytotoxicity persist. In this study, an innovative nanocarrier capable of acid-responsive release of Ag+ and antibiotics is developed, with complete degradation after treatment. Mesoporous organosilica nanoparticle (MON) is encapsulated with hyaluronic acid (HA) to prevent drug leakage and further coated with bacterial outer membrane vesicle (OMV) from Escherichia coli Nissle 1917, creating a nanocarrier with cell-protective capabilities. Ag+ and antibiotic clarithromycin (CLR) are incorporated into the nanocarrier to form CLR-Ag+@MON@HA@OMV (CAMO), designed for the targeted treatment of gastric H. pylori infection. The HA encapsulation ensures acid-responsive release of CLR and Ag+ in the stomach, preventing premature release at non-inflammatory sites. There is a potential for Ag⁺ in CAMO to replace Ni2⁺ at the active site of urease, enhancing the bactericidal effect of CLR through urease inhibition. Furthermore, the OMV provides additional cytoprotection, mitigating cell damage and inflammation response induced by the H. pylori infection. This study introduces a safe and effective nanocarrier that eradicates H. pylori and alleviates gastric inflammation.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Advanced Functional Materials
工程技术-材料科学:综合
CiteScore
29.50
自引率
4.20%
发文量
2086
审稿时长
2.1 months
期刊介绍:
Firmly established as a top-tier materials science journal, Advanced Functional Materials reports breakthrough research in all aspects of materials science, including nanotechnology, chemistry, physics, and biology every week.
Advanced Functional Materials is known for its rapid and fair peer review, quality content, and high impact, making it the first choice of the international materials science community.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


