新型嘧啶和喹唑啉类似物的设计、合成和抗肿瘤评价
IF 6
2区 医学
Q1 CHEMISTRY, MEDICINAL
引用次数: 0
摘要
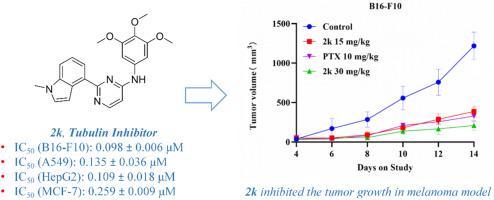
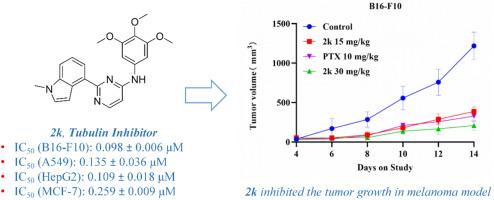
破坏微管动力学已成为一种很有前景的癌症治疗策略。我们设计并合成了新型三甲氧基苯胺基取代嘧啶和喹唑啉衍生物,作为具有抗增殖活性的强效微管抑制剂。化合物 2k 在低纳摩尔浓度下对 B16-F10 癌细胞具有很高的疗效,其 IC50 为 0.098 ± 0.006 μM,与秋水仙碱相当。机理研究发现,2k 能够在体外抑制微管蛋白聚合,导致细胞周期停滞和细胞凋亡。此外,2k 还能抑制肿瘤细胞的迁移,并在黑色素瘤模型中显示出显著的抗肿瘤功效,且无明显毒性。总之,嘧啶衍生物 2k 具有优异的抗癌活性,为新型微管抑制剂的开发提供了新的支架,值得进一步深入研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Design, synthesis and anti-tumor evaluation of novel pyrimidine and quinazoline analogues
Disrupting microtubule dynamics has emerged as a promising strategy for cancer therapy. Novel trimethoxyanilino-substituted pyrimidine and quinazoline derivatives were designed and synthesized to serve as potent microtubule-inhibiting agents with anti-proliferative activity. Compound 2k demonstrates high efficacy against B16–F10 cancer cells at low nanomolar concentrations, with an IC50 of 0.098 ± 0.006 μM, which is comparable to colchicine. Mechanistic studies have revealed that 2k has the ability to inhibit microtubule protein polymerization in vitro, resulting in cell cycle arrest and apoptosis. Furthermore, 2k inhibits tumor cell migration and exhibits significant anti-tumor efficacy in a melanoma tumor model without causing obvious toxicity. In summary, the pyrimidine derivative 2k exhibits excellent anticancer activity and provides a new scaffold for the development of novel microtubule inhibitors, which deserves further in-depth research.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
11.70
自引率
9.00%
发文量
863
审稿时长
29 days
期刊介绍:
The European Journal of Medicinal Chemistry is a global journal that publishes studies on all aspects of medicinal chemistry. It provides a medium for publication of original papers and also welcomes critical review papers.
A typical paper would report on the organic synthesis, characterization and pharmacological evaluation of compounds. Other topics of interest are drug design, QSAR, molecular modeling, drug-receptor interactions, molecular aspects of drug metabolism, prodrug synthesis and drug targeting. The journal expects manuscripts to present the rational for a study, provide insight into the design of compounds or understanding of mechanism, or clarify the targets.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


