Cu3.21Bi4.79S9:双金属超离子策略提升了纳离子存储/萃取的超快动力学性能
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
用作钠离子电池阳极的传统金属硫化物因动力学缓慢而受到阻碍,从而限制了其速率性能。以往解决这一问题的尝试主要集中在具有导电框架的纳米结构配置上。然而,这些纳米材料往往存在堆积密度低和纳米颗粒容易团聚的问题,给实际应用带来了巨大挑战。为了克服这些局限性,本研究提出了一种新型双金属超离子阳极材料 Cu3.21Bi4.79S9,它有效地解决了缓慢的动力学和微米级粒度之间的矛盾。通过利用游离铜原子和铋原子产生的空位,这种材料在钠离子插入和提取过程中形成了快速迁移通道,大大降低了钠离子的迁移障碍。微米级 Cu3.21Bi4.79S9 的开发实现了超快充放电能力,在 45 C(15 A g-1)的高速率下循环 4000 次后,可逆容量达到 325.5 mAh g-1。这项工作标志着该领域的重大进展,它为粗粒度材料在高容量和速率性能之间的固有权衡提供了解决方案,减少了下一代高容量负极材料对纳米结构配置的依赖。本文章由计算机程序翻译,如有差异,请以英文原文为准。
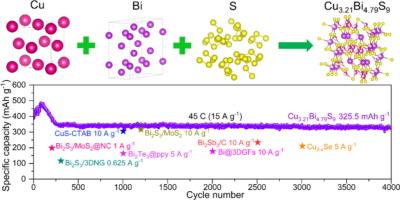
Cu3.21Bi4.79S9: Bimetal superionic strategy boosts ultrafast dynamics for Na-ion storage/extraction
Traditional metal sulfides used as anodes for sodium-ion batteries are hindered by sluggish kinetics, which limits their rate performance. Previous attempts to address this issue focused on nanostructured configurations with conductive frameworks. However, these nanomaterials often suffer from low packing density and the tendency for nanoparticles to agglomerate, posing significant challenges for practical applications. To overcome these limitations, this study presents a novel bimetal superionic anode material Cu3.21Bi4.79S9, which effectively resolves the conflict between sluggish kinetics and micrometer-scale particle size. By leveraging the vacancies created by free Cu and Bi atoms, this material forms rapid migration channels during sodium insertion and extraction, significantly reducing the migration barriers for sodium ions. The development of micrometer-scale Cu3.21Bi4.79S9 enables ultrafast charging-discharging capabilities, achieving a reversible capacity of 325.5 mAh g−1 after 4000 cycles at a high rate of 45 C (15 A g−1). This work marks a significant advancement in the field by offering a solution to the inherent trade-off between high capacity and rate performance in coarse-grained materials, reducing the need for reliance on nanostructured configurations for next-generation high-capacity anode materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


