乙烷在沥青中的溶出和溶解动力学
IF 5
2区 工程技术
Q1 ENGINEERING, MECHANICAL
International Journal of Heat and Mass Transfer
Pub Date : 2024-11-08
DOI:10.1016/j.ijheatmasstransfer.2024.126413
引用次数: 0
摘要
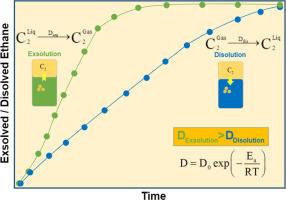
气体的溶解和外溶在许多工程应用中都很常见。当平衡条件受到破坏时,气液界面上就会出现溶解和外溶现象。本研究介绍了乙烷和沥青(一种粘性液体)系统在 80-140 °C 温度范围和 0.69 和 0.35 兆帕压差下的溶出和溶解动力学实验测量数据。根据乙烷/沥青体系的测量数据,采用分析模型估算了外溶系数和溶解系数。在压力差为 0.69 兆帕时,外溶解和溶解过程的扩散值分别为 (2.65-10.48) × 10-8 m2/s 和 (0.73-6.18) × 10-9 m2/s;在压力差为 0.35 兆帕时,外溶解和溶解过程的扩散值分别为 (1.61-8.33) × 10-8 m2/s 和 (0.89-10.78) × 10-9 m2/s。在这两种压差下,乙烷/沥青体系中的外溶动力学均快于溶解动力学。使用阿伦尼乌斯方程计算出的较高的外溶过程活化能也证实了这一点。这些结果为气体外溶解和溶解动力学提供了宝贵的见解,可用于设计和优化非平衡气体和液体接触的过程。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Kinetics of ethane exsolution and dissolution in bitumen
Dissolution and exsolution of gases are common in many engineering applications. Dissolution and exsolution occur across a gas-liquid interface when the equilibrium condition is disturbed. This study presents experimentally measured data on the exsolution and dissolution kinetics of ethane and bitumen (a viscous liquid) system across a temperature range of 80–140 °C and pressure differences of 0.69 and 0.35 MPa. Analytical models were adopted to estimate the exsolution and dissolution coefficients from the measured data for the ethane/bitumen system. The diffusivity values for the exsolution and dissolution processes were estimated to range from (2.65–10.48) × 10−8 m2/s and (0.73–6.18) × 10−9 m2/s, respectively, at a pressure difference of 0.69 MPa, and from (1.61–8.33) × 10−8 m2/s and (0.89–10.78) × 10−9 m2/s, respectively, at a pressure difference of 0.35 MPa. For both pressure differences, the exsolution kinetics were shown to be faster than dissolution in the ethane/bitumen system. This was also confirmed by higher activation energy for the exsolution process calculated using the Arrhenius equation. The results offer valuable insights into the kinetics of gas exsolution and dissolution, with applications in designing and optimizing processes where nonequilibrated gases and liquids are brought into contact.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
10.30
自引率
13.50%
发文量
1319
审稿时长
41 days
期刊介绍:
International Journal of Heat and Mass Transfer is the vehicle for the exchange of basic ideas in heat and mass transfer between research workers and engineers throughout the world. It focuses on both analytical and experimental research, with an emphasis on contributions which increase the basic understanding of transfer processes and their application to engineering problems.
Topics include:
-New methods of measuring and/or correlating transport-property data
-Energy engineering
-Environmental applications of heat and/or mass transfer

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


