通过铂-氧-镍质子和电子 "高速公路 "的微动力调制实现 pH 值通用氢进化
IF 13.1
1区 化学
Q1 Energy
引用次数: 0
摘要
优化碱性和中性条件下的微动力学是开发 pH 值通用型氢进化(HER)电催化剂的一项重要而又具有挑战性的任务。在此,我们构建了一种独特的铂-氧-镍桥,以改变铂纳米粒子(Ptn)和偏磷酸镍(NPO)底物(Pt-NPO)之间的配位和电子环境。从 NPO 到 Ptn 的充分电子传递维持了 Ptn 的富电子环境和低价态。此外,镍位点解离出的 H2O 产生 H*,然后溢出到铂位点结合成 H2,这弥补了铂在 Volmer 步骤中解离 H2O 能力的不足。Pt-NPO 具有长期稳定性,在碱性、中性和酸性介质中分别只需 22.3、33.0 和 30.5 mV 的过电位即可达到 10 mA cm-2。由 Pt-NPO 催化的阴离子交换膜(AEM)水电解槽具有很高的水电解性能,在 80 °C 的 1 M KOH 溶液中,需要 1.73 V 的电池电压才能获得 500 mA cm-2 的电流密度,同时还能在 350 h 内保持良好的稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
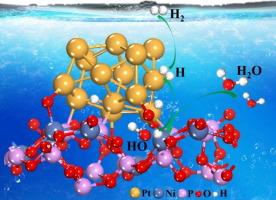
Microdynamic modulation through Pt–O–Ni proton and electron “superhighway” for pH-universal hydrogen evolution
Optimizing the microdynamics in alkaline and neutral conditions is a significant but challenging task in developing pH-universal hydrogen evolution (HER) electrocatalysts. Herein, a unique Pt–O–Ni bridge has been constructed to alter the coordination and electronic environment between Pt nanoparticles (Ptn) and nickel metaphosphate (NPO) substrate (Pt-NPO). Sufficient electron transfer from NPO to Ptn to maintain an electron-rich environment and a low valence state of Ptn. Furthermore, H* is produced from the H2O dissociation on Ni site and then spillover toward Pt sites to bind into H2, which makes up for the insufficient H2O dissociation ability of Pt in Volmer step. Pt-NPO exhibits long-term stability and only need the overpotentials of 22.3, 33.0 and 30.5 mV to attain 10 mA cm−2 in alkaline, neutral and acidic media, respectively. The anion-exchange membrane (AEM) water electrolyzer catalyzed by Pt-NPO shows high water electrolysis performance that a cell voltage of 1.73 V is needed to obtain the current density of 500 mA cm−2 in 1 M KOH at 80 °C, at the same time maintains good stability for 350 h. The regulation strategy proposed in this work is helpful for the design and synthesis of highly efficient pH-universal HER electrocatalysts.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Energy Chemistry
CHEMISTRY, APPLIED-CHEMISTRY, PHYSICAL
CiteScore
19.10
自引率
8.40%
发文量
3631
审稿时长
15 days
期刊介绍:
The Journal of Energy Chemistry, the official publication of Science Press and the Dalian Institute of Chemical Physics, Chinese Academy of Sciences, serves as a platform for reporting creative research and innovative applications in energy chemistry. It mainly reports on creative researches and innovative applications of chemical conversions of fossil energy, carbon dioxide, electrochemical energy and hydrogen energy, as well as the conversions of biomass and solar energy related with chemical issues to promote academic exchanges in the field of energy chemistry and to accelerate the exploration, research and development of energy science and technologies.
This journal focuses on original research papers covering various topics within energy chemistry worldwide, including:
Optimized utilization of fossil energy
Hydrogen energy
Conversion and storage of electrochemical energy
Capture, storage, and chemical conversion of carbon dioxide
Materials and nanotechnologies for energy conversion and storage
Chemistry in biomass conversion
Chemistry in the utilization of solar energy
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


