紫外线增强 Fe2+/PDS 系统降解酸性橙 7:动力学、降解机制和毒性评估
IF 4.1
3区 化学
Q2 CHEMISTRY, PHYSICAL
Journal of Photochemistry and Photobiology A-chemistry
Pub Date : 2024-10-24
DOI:10.1016/j.jphotochem.2024.116054
引用次数: 0
摘要
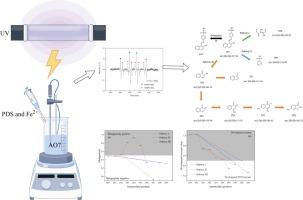
基于Fe2+催化过硫酸盐(PDS)过程的不足,本研究引入了紫外光(UV)协同Fe2+活化PDS系统来降解酸性橙7(AO7)染料。研究了不同初始 pH 值、PDS 用量、Fe2+ 用量和不同紫外线强度对 AO7 降解速率的影响,并确定了表观反应速率的动力学模型。结果表明,紫外线/Fe2+/PDS 系统能在较宽的 pH 值范围内有效降解 AO7。在最佳条件下,10 分钟内 AO7 的去除率达到 96%。讨论了不同无机阴离子对 AO7 的影响;Cl- 对降解效果影响很小,SO42-、NO3- 和 HCO3- 在不同程度上抑制了该系统对 AO7 的降解。通过淬灭实验和 EPR 捕捉,确定了反应中活性自由基(SO4-和-OH)的存在,并进一步研究了它们对降解的贡献。通过 LC-MS/MS 检测到了 AO7 的九种中间产物,并结合紫外可见光谱推断了 AO7 的降解途径。最后,利用 Toxtree 和 T.E.S.T 毒性预测模型对 AO7 中间体的生态毒性和三致效应进行了评估,结果表明最终降解产物的毒性无突变性、无致畸性、无遗传性和非遗传性致癌性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
UV-enhanced Fe2+/PDS system for degradation of acid orange 7: Kinetics, degradation mechanism and toxicity assessment
Based on the deficiency of the Fe2+ catalyzed persulfate (PDS) process, ultraviolet (UV) synergistic Fe2+ activated PDS system was introduced in this study for the degradation of acid orange 7 (AO7) dye. The effects of different initial pH, PDS dosage, Fe2+ dosage, and different UV intensities on the degradation rate of AO7 were investigated, and a kinetic model for the apparent reaction rate was determined. The results showed that the UV/Fe2+/PDS system was effective in degrading AO7 in a wide pH range. Under optimal conditions, 96 % removal of AO7 was achieved in 10 min. The effects of different inorganic anions on AO7 were discussed; Cl− had little effect on the degradation effect, and SO42−, NO3− and HCO3− inhibited the degradation of AO7 by the system to varying degrees. The presence of reactive radicals (SO4·− and·OH) in the reaction was determined by quenching experiments and EPR capture and their degradation contribution was further investigated. Nine intermediates of AO7 were detected by LC-MS/MS, and the degradation pathway of AO7 was inferred by combining with UV-Vis spectra. Finally, the ecotoxicity and tritogenic effects of AO7 intermediates were assessed by Toxtree and T.E.S.T toxicity prediction models, which showed that the final degradation product toxicity was non-mutagenic, non-teratogenic, and devoid of hereditary and non-hereditary carcinogenicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.90
自引率
7.00%
发文量
580
审稿时长
48 days
期刊介绍:
JPPA publishes the results of fundamental studies on all aspects of chemical phenomena induced by interactions between light and molecules/matter of all kinds.
All systems capable of being described at the molecular or integrated multimolecular level are appropriate for the journal. This includes all molecular chemical species as well as biomolecular, supramolecular, polymer and other macromolecular systems, as well as solid state photochemistry. In addition, the journal publishes studies of semiconductor and other photoactive organic and inorganic materials, photocatalysis (organic, inorganic, supramolecular and superconductor).
The scope includes condensed and gas phase photochemistry, as well as synchrotron radiation chemistry. A broad range of processes and techniques in photochemistry are covered such as light induced energy, electron and proton transfer; nonlinear photochemical behavior; mechanistic investigation of photochemical reactions and identification of the products of photochemical reactions; quantum yield determinations and measurements of rate constants for primary and secondary photochemical processes; steady-state and time-resolved emission, ultrafast spectroscopic methods, single molecule spectroscopy, time resolved X-ray diffraction, luminescence microscopy, and scattering spectroscopy applied to photochemistry. Papers in emerging and applied areas such as luminescent sensors, electroluminescence, solar energy conversion, atmospheric photochemistry, environmental remediation, and related photocatalytic chemistry are also welcome.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


