用于化学战剂解毒的氢氧化锆活性碳杂化材料:水和温度的影响
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
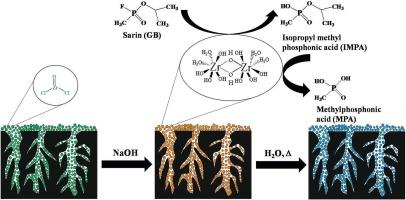
鉴于近期化学战剂(CWAs)的威胁,科学界广泛关注开发基于无害环境技术的有效、安全的 CWAs 去污方法,并避免使用腐蚀性和有毒化学品。在此,我们报告了 CWAs 沙林和硫芥在混合材料氢氧化锆-粒状活性炭(即 Zr(OH)4@GAC)上的分解情况,该材料是利用活性氢氧化锆和高比表面积碳合成的。通过改变前驱体(即氧氯化锆)的浓度,在 GAC 的孔隙中就地生成氢氧化锆,然后进行水解。采用粉末 X 射线衍射、TGA、傅立叶变换红外光谱、BET、扫描电镜、EDX 和 TEM 等分析技术对反应性杂化材料 Zr(OH)4@GAC 的形态、结构和纹理特性进行了研究。此外,还评估了 Zr(OH)4@GAC 在水解消减化学战剂沙林和硫芥中的降解能力。在原始实验室条件下,活性杂化材料 Zr(OH)4@GAC 的有效性归因于 Zr(OH)4 的缺陷和多种表面羟基以及高比表面积碳基体的结合。此外,还研究了含水量和温度对 CWAs 降解的影响,将含水量从 2% 改为 8%,温度从 25 °C 改为 45 °C。结果表明,降解过程遵循一阶反应动力学。观察发现,含水量越高,温度越高,Zr(OH)4@GAC 上 CWA 的分解速度越快;相反,温度越低,CWA 的降解速度越慢。混合材料 Zr(OH)4@GAC 对 CWAs 去污能力的显著增强归因于 GAC(吸附能力)与 Zr(OH)4 的活性官能团的协同效应。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Zirconium hydroxide-activated carbon hybrid material for chemical warfare agents detoxification: Implication of water and temperature
In light of the recent threat from chemical warfare agents (CWAs), the scientific community has focused extensively on developing effective and safe decontamination methodology for CWAs based on environmentally benign technology and the avoidance of corrosive and toxic chemicals. Herein, we report the decomposition of CWAs sarin and sulphur mustard on the hybrid material Zirconium hydroxide-granular activated carbon i.e. Zr(OH)4@GAC which is synthesized by utilizing the reactive zirconium hydroxide and high surface area carbon. In-situ zirconium hydroxide was generated in the pores of GAC by varying the precursor concentration i.e. zirconium oxychloride followed by hydrolysis. The morphology, structural, and textural properties of the reactive hybrid material Zr(OH)4@GAC were examined using several analytical techniques including powder x-ray diffraction, TGA, FT-IR, BET, SEM, EDX, and TEM. Furthermore, the degradation capability of Zr(OH)4@GAC was evaluated in the hydrolytic abatement of CWAs sarin and sulphur mustard. Under pristine laboratory conditions, the effectiveness of reactive hybrid material Zr(OH)4@GAC has been attributed due to a combination of defects and diverse surface hydroxyl species of Zr(OH)4, as well as a high surface area carbon matrix. The impact of water content and temperature on CWAs degradation was also investigated by altering the water percentage from 2 to 8 % and the temperature 25 °C to 45 °C. The GC–MS/GC technique was used to observe the kinetics of in-situ degradation of CWAs over Zr(OH)4@GAC The results indicated that the degradation process follows a first-order reaction kinetics. It is observed that higher water content along with elevated temperature enhance CWA decomposition on Zr(OH)4@GAC; conversely, at lower temperature, it slowed down the degradation of CWAs. This significant enhancement in the decontamination capability of hybrid materials Zr(OH)4@GAC towards CWAs was attributed to the synergistic effects of GAC (adsorption capacity) coupled with the reactive functional group of Zr(OH)4.This strategy will pave the way for the development of self-detoxifying adsorbent material for environmental and defence purposes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


