一锅合成用于氧进化反应电催化的石墨烯纳米片-镍钴 LDHs 纳米复合材料
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, COATINGS & FILMS
引用次数: 0
摘要
众所周知,氧进化反应(OER)因其缓慢的动力学而成为整个水分离过程的瓶颈。在这方面,构建一种高性能、高性价比的电极材料可能会改变游戏规则。在此,我们采用简单的溶热法制备了一种由石墨烯纳米片和镍钴层状双氢氧化物(NiCo LDH)组成的 2D/2D 纳米复合材料。从场发射扫描(FE-SEM)和透射(TEM)电子显微镜图像中可以观察到附着在石墨烯纳米片上的镍钴层状双氢氧化物的二维花状结构。X 射线衍射(XRD)图显示了 Ni(OH)2 和 Co(OH)2 晶格以及六角形石墨烯晶体的形成。能量色散 X 射线光谱(EDS)显示,在石墨烯/镍钴 LDH 纳米复合材料中,镍和钴的比例为 2.2:1。X 射线元素图显示了样品中元素的均匀分布,并在傅立叶变换红外光谱(FTIR)和拉曼光谱中观察到了相应的官能团。X 射线光电子能谱(XPS)确定了纳米复合材料中二价和三价金属的共存。N2 吸附-解吸研究表明,纳米复合材料具有高比表面积的狭缝形离子可进入微孔和中孔。这有利于改性电极在电解液中的离子扩散过程。石墨烯/镍钴 LDH 纳米复合材料的起始电位为 1.56 V,10 mA cm-2 时的过电位为 338 mV,塔菲尔斜率为 69 mV dec-1,电荷转移电阻 (Rct) 为 27 Ω,双层电容 (Cdl) 为 24 μF,电化学活性表面积 (ECSA) 为 0.6 cm2,粗糙度系数 (RF) 为 20。在 OER 电位下连续测量 10 小时后,电极仍能保持 98.3% 的初始信号。这种电催化活性得益于石墨烯纳米片和镍钴低密度氧化物之间的建设性协同作用所产生的片状形态、通过层间空间的有效氢氧根离子转移、增强的导电性和高化学稳定性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
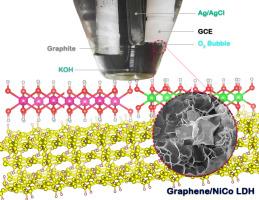
One-pot synthesis of graphene nanosheets‑nickel cobalt LDHs nanocomposite for electrocatalysis of oxygen evolution reaction
Oxygen evolution reaction (OER) is known as a bottleneck for the overall water-splitting process due to its slow kinetics. In this regard, constructing a high-performance, cost-effective electrode material may act as a game changer. Here, a 2D/2D nanocomposite has prepared out of graphene nanosheets and nickel‑cobalt layered double hydroxide (NiCo LDH) by a simple solvothermal method. The 2D flower-like structures of NiCo LDH attached to graphene nanosheets have observed in field-emission scanning (FE-SEM) and transmission (TEM) electron microscopy images. The X-ray diffraction (XRD) patterns have revealed the formation of Ni(OH)2 and Co(OH)2 lattices along with hexagonal graphene crystals. The energy-dispersive X-ray spectroscopy (EDS) has noted the 2.2:1 ratio of nickel to cobalt in the graphene/NiCo LDH nanocomposite. X-ray elemental maps have shown the uniform distribution of elements in the sample, and the corresponding functional groups have observed in the Fourier-transform infrared (FTIR) and Raman spectra. X-ray photoelectron spectroscopy (XPS) has determined the coexistence of divalent and trivalent metals in the nanocomposite. The N2 adsorption-desorption study has shown signs of slit-shaped ion-accessible micro and mesopores with high specific surface area for the nanocomposite. It has benefited the ion diffusion process during the exposure of the modified electrode to the electrolyte. The graphene/NiCo LDH nanocomposite has provided the onset potential of 1.56 V, overpotential of 338 mV at 10 mA cm−2, Tafel slope of 69 mV dec−1, charge-transfer resistance (Rct) of 27 Ω, double layer capacitance (Cdl) of 24 μF, electrochemically active surface area (ECSA) of 0.6 cm2, and roughness factor (RF) of 20. The electrode has maintained 98.3 % of its initial signal after 10 h continuous measurement at OER potential. This electrocatalytic activity refers to the sheet-like morphology, effective hydroxide ion transfer through interlayer spaces, enhanced conductivity, and high chemical stability achieved by a constructive synergy between graphene nanosheets and NiCo LDH.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Diamond and Related Materials
工程技术-材料科学:综合
CiteScore
6.00
自引率
14.60%
发文量
702
审稿时长
2.1 months
期刊介绍:
DRM is a leading international journal that publishes new fundamental and applied research on all forms of diamond, the integration of diamond with other advanced materials and development of technologies exploiting diamond. The synthesis, characterization and processing of single crystal diamond, polycrystalline films, nanodiamond powders and heterostructures with other advanced materials are encouraged topics for technical and review articles. In addition to diamond, the journal publishes manuscripts on the synthesis, characterization and application of other related materials including diamond-like carbons, carbon nanotubes, graphene, and boron and carbon nitrides. Articles are sought on the chemical functionalization of diamond and related materials as well as their use in electrochemistry, energy storage and conversion, chemical and biological sensing, imaging, thermal management, photonic and quantum applications, electron emission and electronic devices.
The International Conference on Diamond and Carbon Materials has evolved into the largest and most well attended forum in the field of diamond, providing a forum to showcase the latest results in the science and technology of diamond and other carbon materials such as carbon nanotubes, graphene, and diamond-like carbon. Run annually in association with Diamond and Related Materials the conference provides junior and established researchers the opportunity to exchange the latest results ranging from fundamental physical and chemical concepts to applied research focusing on the next generation carbon-based devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


