创新型医用聚(乙二醇-三亚甲基碳酸酯-ε-己内酯)缓释植入物的降解和体内评估
IF 5.8
2区 化学
Q1 POLYMER SCIENCE
引用次数: 0
摘要
延迟释放植入物是一种在特定滞后时间后释放治疗药物的潜在方法。通过浸涂法制造的储层植入物可以方便地装载有效载荷,设计为皮下注射,释放量由可生物降解的软性三元共聚物--聚(乙二醇-共三亚甲基碳酸酯-共ε-己内酯)的物理化学特性控制。由于三元共聚物降解微乎其微,阻碍了有效载荷的释放,因此出现了一个滞后期(第 1 阶段)。到 37 天时(第 2 阶段),三元共聚物的大量侵蚀达到有效载荷开始向周围介质扩散的状态,占释放量的 75% 和质量损失的 20%,表明扩散和侵蚀介导的释放相结合。最后,第 3 阶段主要由扩散控制,有效载荷的释放量为 20%,聚合物的质量损失极小。在啮齿动物临床前模型中,三元共聚物与宿主组织很好地结合在一起,异物反应平衡。这项研究证明了使用一种独特的医用级聚酯聚合物来开发缓释植入物的可行性,具有很好的应用前景。这种装置的应用前景包括输送敏感的有效载荷,如蛋白质疫苗,因为在制造过程中避免了聚合物与有效载荷之间的相互作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
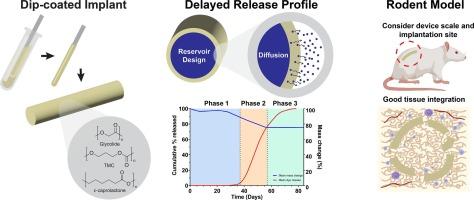
Degradation and in vivo evaluation of an innovative delayed release implant of medical grade poly(glycolide-co-trimethylene carbonate-co-ε-caprolactone)
Delayed release implants are a potential method to deliver a therapeutic after a specified lag time. A reservoir implant fabricated by dip-coating allows facile loading of a payload designed to be injected subcutaneously with release controlled by the physicochemical properties of a soft biodegradable terpolymer, poly(glycolide-co-trimethylene carbonate-co-ε-caprolactone). A triphasic profile is achieved, consisting of a lag period (Phase 1) due to negligible terpolymer degradation preventing payload release. By 37 days (Phase 2) bulk erosion of the terpolymer reaches a state where payload begins to diffuse into the surrounding medium, accounting for 75 % of release and 20 % mass loss, indicating a combination of diffusion and erosion-mediated release. Lastly, Phase 3 is predominately diffusion-controlled as 20 % payload release is achieved with minimal mass loss of the polymer. In a rodent preclinical model, the terpolymer was well-integrated within host tissue with a balanced foreign body reaction. This study demonstrates the feasibility of using a unique medical grade poly(ester)-based polymer to develop a delayed release implant with excellent potential for translation. Prospective applications of this device include the delivery of sensitive payloads such as protein vaccines as polymer-payload interactions during manufacturing are avoided.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

European Polymer Journal
化学-高分子科学
CiteScore
9.90
自引率
10.00%
发文量
691
审稿时长
23 days
期刊介绍:
European Polymer Journal is dedicated to publishing work on fundamental and applied polymer chemistry and macromolecular materials. The journal covers all aspects of polymer synthesis, including polymerization mechanisms and chemical functional transformations, with a focus on novel polymers and the relationships between molecular structure and polymer properties. In addition, we welcome submissions on bio-based or renewable polymers, stimuli-responsive systems and polymer bio-hybrids. European Polymer Journal also publishes research on the biomedical application of polymers, including drug delivery and regenerative medicine. The main scope is covered but not limited to the following core research areas:
Polymer synthesis and functionalization
• Novel synthetic routes for polymerization, functional modification, controlled/living polymerization and precision polymers.
Stimuli-responsive polymers
• Including shape memory and self-healing polymers.
Supramolecular polymers and self-assembly
• Molecular recognition and higher order polymer structures.
Renewable and sustainable polymers
• Bio-based, biodegradable and anti-microbial polymers and polymeric bio-nanocomposites.
Polymers at interfaces and surfaces
• Chemistry and engineering of surfaces with biological relevance, including patterning, antifouling polymers and polymers for membrane applications.
Biomedical applications and nanomedicine
• Polymers for regenerative medicine, drug delivery molecular release and gene therapy
The scope of European Polymer Journal no longer includes Polymer Physics.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


