从水杨醛合成 (2,3-二氢苯并呋喃-3-基)乙酸:三甲基硅作为无踪活化基团
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
水杨醛很容易与(氯甲基)三甲基硅烷发生烷基化反应,从而得到硅烷化的邻甲氧基苯甲醛,收率为 85-96%。这些醛以极高的收率转化为亚芳基丙二酸酯。在 140 °C 的 DMF 溶液中,在氟化铯的促进下,O-(三甲基硅烷基)甲基被用作芳氧基甲基阴离子的合成等价物,在烯分子上发生亲核环化反应。通过这种方法,以水杨醛为原料合成了(2,3-二氢苯并呋喃-3-基)乙酸和相应的酯,总产率为 41-70%。本文章由计算机程序翻译,如有差异,请以英文原文为准。
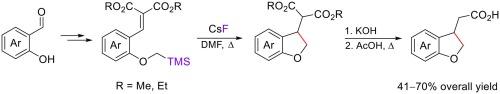
Synthesis of (2,3-dihydrobenzofuran-3-yl)acetic acids from salicylic aldehydes: Trimethylsilyl as traceless activating group
Salicylic aldehydes were readily alkylated with (chloromethyl)trimethylsilane to give the silylated ortho-methoxy benzaldehydes in 85–96 % yields. These aldehydes were converted into arylidenemalonates in excellent yields. O-(Trimethylsilyl)methyl group was applied as the synthetic equivalent of aryloxymethyl anion in the nucleophilic cyclization at the alkene moiety promoted by cesium fluoride in DMF at 140 °C. In this way, (2,3-dihydrobenzofuran-3-yl)acetic acids and the corresponding esters were synthesized from salicylic aldehydes in 41–70 % overall yields.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


