镍催化下芳基氟化物与烷基溴的脱羰基还原烷基化机理的 DFT 研究
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
通过密度泛函理论计算,研究了镍催化下芳基氟化物与烷基溴的脱羰基还原烷基化反应机理。计算结果表明,反应机理包括 C-F 键氧化加成、脱羰基、烷基自由基加成、C(sp2)-C(sp3)还原消除生成产物、单电子转移(SET)和 Zn 还原以再生引发复合物等连续步骤。结果发现,C-F 键氧化加成步骤是决定速率的步骤。脱羰基作用应发生在烷基自由基加成之前。此外,还分析了 1,3-双(二苯基膦)丙烷(DPPP)配体和不同底物对反应活性的影响。这些计算结果揭示了详细的反应机理,并揭示了实验中一些不明确的建议。本文章由计算机程序翻译,如有差异,请以英文原文为准。
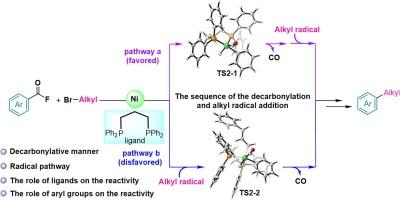
DFT study on mechanism of nickel-catalyzed decarbonylative reductive alkylation of aroyl fluorides with alkyl bromides
The mechanism of the nickel-catalyzed decarbonylative reductive alkylation of aroyl fluorides with alkyl bromides is investigated using density functional theory calculations. The calculation result shows that the reaction mechanism involves sequential steps of C–F bond oxidative addition, decarbonylation, alkyl radical addition, C(sp2)–C(sp3) reductive elimination to afford the product, single electron transfer (SET) and reduction by Zn to regenerate the initiating complex. And the step of C–F bond oxidative addition was found to be the rate-determining step. The decarbonylation should occur before the alkyl radical addition. The effects of 1,3-bis(diphenylphosphino)propane (DPPP) ligand and different substrates on the reactivity were also analyzed. These calculation results disclosed the detailed reaction mechanism and shed lights on some ambiguous suggestions from experiments.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


