由 2-甲磺酰基-5-硝基苯甲醛衍生的肼类化合物的合成、晶体结构、细胞毒性(MCF-7 和 HeLa)和自由基清除活性
IF 2.5
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
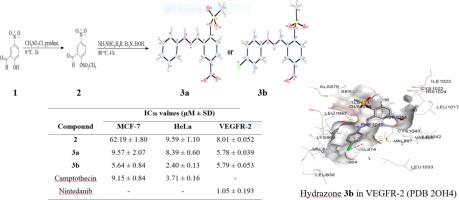
妇女乳腺癌和宫颈癌发病率的上升已成为公共卫生问题,给全球,尤其是中低收入国家造成了沉重的经济负担。这就需要寻找新的、有效的、副作用小或无副作用的抗癌药物。研究人员采用光谱和单晶 X 射线衍射 (XRD) 技术,对从 2-甲酰基-4-硝基苯甲磺酸盐中提取的酰肼进行了表征。然后,在体外评估了这些化合物对人类乳腺腺癌(MCF-7)和人类宫颈癌(HeLa)细胞系的抗生长作用,以及对非洲绿猴肾(Vero)细胞系的细胞毒性。与喜树碱(IC50 = 9.15 ± 0.84 µM 和 3.71 ± 0.16 µM)相比,3b 的苯腙分子对位上存在一个氯原子,导致其对 MCF-7 和 HeLa 细胞系的细胞毒性增加,IC50 值分别为 5.64 ± 0.84 µM 和 2.40 ± 0.13 µM。与抗癌药物多柔比星(IC50 = 0.78 ± 0.04 µM)和宁替达尼(IC50 = 0.24 ± 0.02 µM)相比,化合物 2、3a 和 3b 对 Vero 细胞的毒性明显降低,IC50 值分别为 17.86 ± 1.12 µM、11.89 ± 1.01 µM 和 24.42 ± 0.70 µM。与甲醛前体 2(IC50 = 8.01 ± 0.052 µM)相比,酰腙 3a 和 3b 对血管内皮生长因子受体-2(VEGFR-2)酪氨酸激酶具有很强的抑制作用(IC50 = 5.78 ± 0.039 µM和 5.79 ± 0.053 µM),但与宁替达尼(IC50 = 1.05 ± 0.193 µM)相比,活性较低。与抗坏血酸和母体 2-甲酰基-4-硝基苯甲磺酸盐相比,腙衍生物具有显著的 2,2-二苯基-1-苦基肼(DPPH)自由基清除活性。硅学分子对接研究揭示了这些酰肼在血管内皮生长因子受体-2 酪氨酸激酶和细胞色素 c 过氧化物酶活性位点的结合潜力。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Synthesis, crystal structure, cytotoxicity (MCF-7 and HeLa) and free radical scavenging activity of the hydrazones derived from 2-methylsulfonyl-5-nitrobenzaldehyde
The rising levels of breast and cervical cancers among women have become public health problem with high economic burden globally and more especially in low- and middle-income countries. This necessitates discoving new and potent anticancer drugs with reduced or no side effects. The hydrazones derived from 2-formyl-4-nitrophenyl methanesulfonate were characterized using a combination of spectroscopic and single-crystal X-ray diffraction (XRD) techniques. The compounds were, in turn, evaluated for antigrowth effect in vitro against the human breast adenocarcinoma (MCF-7) and human cervical cancer (HeLa) cell lines and for cytotoxicity against the African Green Monkey kidney (Vero) cell line. The presence of a chlorine atom on the para position of the phenylhydrazone moiety of 3b resulted in increased cytotoxicity against the MCF-7 and the HeLa cell lines compared to camptothecin (IC50 = 9.15 ± 0.84 µM and 3.71 ± 0.16 µM, respectively) with IC50 values of 5.64 ± 0.84 µM and 2.40 ± 0.13 µM, respectively. Compounds 2, 3a and 3b were found to exhibit significantly reduced toxicity against the Vero cells compared to the anti-cancer drugs, doxorubicin (IC50 = 0.78 ± 0.04 µM) and nintedanib (IC50 = 0.24 ± 0.02 µM) with the IC50 values of 17.86 ± 1.12, 11.89 ± 1.01 and 24.42 ± 0.70 µM, respectively. The hydrazones 3a and 3b exhibited a strong inhibitory effect against the vascular endothelial growth factor receptor-2 (VEGFR-2) tyrosine kinase (IC50 = 5.78 ± 0.039 μM and 5.79 ± 0.053 µM, respectively) compared to the carbaldehyde precursor 2 (IC50 = 8.01 ± 0.052 µM) though less active compared to nintedanib (IC50 = 1.05 ± 0.193 µM). The hydrazone derivatives exhibited significant 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity compared to ascorbic acid and the parent 2-formyl-4-nitrophenylmethanesulfonate. In silico molecular docking studies revealed the binding potential of the hydrazones at the active site of VEGFR-2 tyrosine kinase and cytochrome c peroxidase.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


