β-酮胺共价有机框架纳米平台结合光动力免疫疗法的免疫检查点阻断抑制胶质母细胞瘤进展
IF 18
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
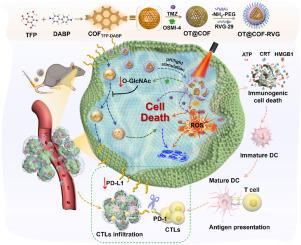
光动力免疫疗法与内源性清除PD-L1免疫检查点阻断疗法相结合的协同方法有望提高胶质母细胞瘤(GBM)患者的生存率。在肿瘤中观察到的O-GlcNAc糖酵解上调可能有助于稳定内源性PD-L1蛋白,从而促进肿瘤免疫逃避。本研究提出了一种与 pH 值相适应的激发态分子内质子转移(ESIPT)异构化β-酮酰胺基共价有机框架(COF)纳米平台(简称 OT@COF-RVG)。将替莫唑胺(TMZ)和OSMI-4(O-GlcNAc转移酶抑制剂)整合到COF空腔中,然后用聚乙二醇和狂犬病毒肽RVG-29对其表面进行修饰,显示了TMZ化疗增敏和启动光动力疗法(PDT)的潜力。通过抑制 O-GlcNAc 和促进 PD-L1 的溶酶体降解,OT@COF-RVG 增强了免疫检查点阻断疗法(ICB)的效果。此外,用 OT@COF-RVG 治疗会导致活性氧(ROS)水平显著升高,从而重建免疫刺激状态,诱导免疫原性细胞死亡(ICD)。总之,我们的研究揭示了 GBM 中的 O-GlcNAc 与肿瘤逃避免疫反应之间的相关性,同时展示了 OT@COF-RVG 在重塑 GBM 免疫抑制微环境方面的潜力,并为临床免疫疗法提供了一种更有效的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
β-Ketoenamine covalent organic framework nanoplatform combined with immune checkpoint blockade via photodynamic immunotherapy inhibit glioblastoma progression
The synergistic approach of combining photodynamic immunotherapy with endogenous clearance of PD-L1 immune checkpoint blockade therapy holds promise for enhancing survival outcomes in glioblastoma (GBM) patients. The observed upregulation of O-GlcNAc glycolysis in tumors may contribute to the stabilization of endogenous PD-L1 protein, facilitating tumor immune evasion. This study presents a pH-adapted excited state intramolecular proton transfer (ESIPT)-isomerized β-ketoamide-based covalent organic framework (COF) nanoplatform (denoted as OT@COF-RVG). Temozolomide (TMZ) and OSMI-4 (O-GlcNAc transferase inhibitor) were integrated into COF cavities, then modified on the surface with polyethylene glycol and the rabies virus peptide RVG-29, showing potential for sensitizing TMZ chemotherapy and initiating photodynamic therapy (PDT). By inhibiting O-GlcNAc and promoting lysosomal degradation of PD-L1, OT@COF-RVG enhanced the effectiveness of immune checkpoint blockade (ICB) therapy. Additionally, treatment with OT@COF-RVG led to a notable elevation in reactive oxygen species (ROS) levels, thereby re-establishing an immunostimulatory state, inducing immunogenic cell death (ICD). In summary, our research unveiled a correlation between O-GlcNAc in GBM and the evasion of immune responses by tumors, while showcasing the potential of OT@COF-RVG in reshaping the immunosuppressive microenvironment of GBM and offering a more effective approach to immunotherapy in clinical settings.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Bioactive Materials
Biochemistry, Genetics and Molecular Biology-Biotechnology
CiteScore
28.00
自引率
6.30%
发文量
436
审稿时长
20 days
期刊介绍:
Bioactive Materials is a peer-reviewed research publication that focuses on advancements in bioactive materials. The journal accepts research papers, reviews, and rapid communications in the field of next-generation biomaterials that interact with cells, tissues, and organs in various living organisms.
The primary goal of Bioactive Materials is to promote the science and engineering of biomaterials that exhibit adaptiveness to the biological environment. These materials are specifically designed to stimulate or direct appropriate cell and tissue responses or regulate interactions with microorganisms.
The journal covers a wide range of bioactive materials, including those that are engineered or designed in terms of their physical form (e.g. particulate, fiber), topology (e.g. porosity, surface roughness), or dimensions (ranging from macro to nano-scales). Contributions are sought from the following categories of bioactive materials:
Bioactive metals and alloys
Bioactive inorganics: ceramics, glasses, and carbon-based materials
Bioactive polymers and gels
Bioactive materials derived from natural sources
Bioactive composites
These materials find applications in human and veterinary medicine, such as implants, tissue engineering scaffolds, cell/drug/gene carriers, as well as imaging and sensing devices.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


