克服肿瘤缺氧铋基三元异质结实现缺陷调制增强肿瘤声催化免疫疗法
IF 12.8
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
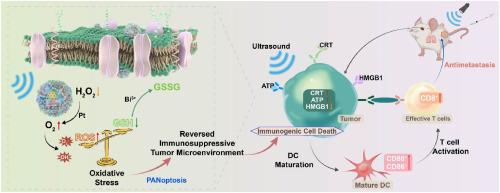
通过声催化作用诱导活性氧(ROS)以启动炎性程序性细胞死亡(PANoptosis)和免疫原性细胞死亡(ICD),是一种很有前景的可激活癌症免疫疗法策略。然而,超声波敏化剂在超声波下产生的 ROS 有限,以及免疫抑制性肿瘤微环境阻碍了超声免疫疗法的效率。为了克服这些挑战,我们开发了一种铋基三元异质结 Bi@Bi2O3-Pt-PEG(BBOP),用于声催化疗法,旨在激活免疫反应。该系统能在声催化过程中增强 ROS 的产生,并利用诱导 PANoptosis 和 ICD 的双重治疗机制来提高抗肿瘤疗效。BBOP 通过形成中间 Bi2O3 层和引入铂形成 Z 型异质结和肖特基接触。这些结构大大提高了声催化活性,而铂纳米酶则表现出类似催化剂的行为,为声催化提供氧气,促进 ROS 生成,有效缓解肿瘤缺氧,从而减少免疫抑制。进一步的体外和体内实验证实,BBOP 能够在超声下激活免疫反应,抑制肿瘤生长和转移。RNA 测序揭示了其治疗生物学机制。该催化系统的构建不仅为优化声波增敏剂提供了启示,也为临床癌症治疗提供了更安全有效的声波免疫疗法激活策略和理论依据。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Overcoming tumor hypoxic bismuth-based ternary heterojunctions enable defect modulation-augmented tumor sonocatalytic immunotherapy
Inducing reactive oxygen species (ROS) via sonocatalysis to initiate inflammatory programmed cell death (PANoptosis) and immunogenic cell death (ICD) presents a promising strategy for activatable cancer immunotherapy. However, the limited ROS generation by sonosensitizers under ultrasound and the immunosuppressive tumor microenvironment hinder the efficiency of sono-immunotherapy. To overcome these challenges, a bismuth-based ternary heterojunction, Bi@Bi2O3–Pt-PEG (BBOP), was developed for sonocatalytic therapy aimed at activating immune responses. This system enhances ROS production during sonocatalysis and leverages dual therapeutic mechanisms by inducing PANoptosis and ICD to achieve improved anti-tumor efficacy. BBOP forms a Z-scheme heterojunction and Schottky contact through the formation of an intermediate Bi2O3 layer and the introduction of Pt. These structures significantly enhance sonocatalytic activity, while the Pt nanozyme exhibits catalase-like behavior, supplying oxygen for sonocatalysis, boosting ROS generation, and effectively relieving tumor hypoxia to reduce immune suppression. Further in vitro and in vivo experiments confirmed BBOP's ability to activate immune responses under ultrasound, inhibiting tumor growth and metastasis. RNA sequencing revealed the therapeutic biological mechanisms. The construction of this catalytic system not only provides insights for optimizing sonosensitizers but also offers a safer and more effective sono-immunotherapy activation strategy and theoretical basis for clinical cancer treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Biomaterials
工程技术-材料科学:生物材料
CiteScore
26.00
自引率
2.90%
发文量
565
审稿时长
46 days
期刊介绍:
Biomaterials is an international journal covering the science and clinical application of biomaterials. A biomaterial is now defined as a substance that has been engineered to take a form which, alone or as part of a complex system, is used to direct, by control of interactions with components of living systems, the course of any therapeutic or diagnostic procedure. It is the aim of the journal to provide a peer-reviewed forum for the publication of original papers and authoritative review and opinion papers dealing with the most important issues facing the use of biomaterials in clinical practice. The scope of the journal covers the wide range of physical, biological and chemical sciences that underpin the design of biomaterials and the clinical disciplines in which they are used. These sciences include polymer synthesis and characterization, drug and gene vector design, the biology of the host response, immunology and toxicology and self assembly at the nanoscale. Clinical applications include the therapies of medical technology and regenerative medicine in all clinical disciplines, and diagnostic systems that reply on innovative contrast and sensing agents. The journal is relevant to areas such as cancer diagnosis and therapy, implantable devices, drug delivery systems, gene vectors, bionanotechnology and tissue engineering.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


