两种提取公牛附睾精子方法的比较
IF 1.9
Q2 AGRICULTURE, DAIRY & ANIMAL SCIENCE
引用次数: 0
摘要
为了避免丢失宝贵的遗传物质,例如稀有品种或濒危物种的个体,可能需要提取附睾精子,但提取的精子样本质量可能很差。我们比较了从屠宰场材料中提取公牛附睾精子的两种方法。公牛的年龄为 16-23 个月。提取精子的方法是在附睾尾部切开一厘米长的切口,让精子流出(方法 A),或冲洗附睾尾部(方法 B)。对每头公牛使用这两种方法,交替使用左右附睾,即如果对公牛 1 的左侧附睾使用 A 方法,则对公牛 2 的右侧附睾使用该方法,等等。在 Andromed 中将提取样本中的精子浓度调整为 69 × 106/毫升;将精子样本装入 0.25 毫升吸管中。在 5 °C 下冷却两小时后,将吸管置于液氮上方 4 厘米处 20 分钟,然后将其转移到液氮中。对新鲜样本和解冻后的样本进行精子活力、存活率、活性氧、膜完整性和 DNA 片段分析。两种方法对新鲜精液中所有参数的检测结果均无差异。虽然解冻样本的精子质量低于新鲜样本,但两种提取方法提取的精子质量在解冻样本中没有差异。总之,这两种方法都适用于公牛附睾精子的提取。本文章由计算机程序翻译,如有差异,请以英文原文为准。
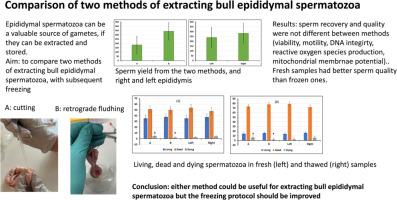
Comparison of two methods of extracting bull epididymal spermatozoa
Extraction of epididymal spermatozoa may be necessary to avoid losing valuable genetic material, for example, from individuals of rare breeds or endangered species, but the resulting sperm samples may be of poor quality. Two methods of extracting bull epididymal spermatozoa from slaughterhouse material were compared. The bulls were 16–23 months of age. Spermatozoa were extracted by making an incision one cm in length in the tail of the epididymis to allow the spermatozoa to flow out (method A), or by flushing the tail of epididymis (method B). The two methods were used for each bull, alternating between right and left epididymis, i.e. if method A was used for the left epididymis in Bull 1, it was used for the right epididymis in bull 2, etc. Sperm concentration in the extracted samples was adjusted to 69 × 106/mL in Andromed; the sperm sample was packed in 0.25 mL straws. After cooling for two h at 5 °C, the straws were placed 4 cm above liquid nitrogen for 20 min before transferring them to liquid nitrogen. Sperm motility, viability, reactive oxygen species, membrane integrity and DNA fragmentation were analysed in the fresh samples and again after thawing. The results for all parameters in fresh semen were not different between methods. Although sperm quality was lower in thawed samples than in fresh samples, there was no difference in sperm quality between the two extraction methods in the thawed samples. In conclusion, both methods are useful for the extraction of bull epididymal spermatozoa.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Veterinary and Animal Science
Veterinary-Veterinary (all)
CiteScore
3.50
自引率
0.00%
发文量
43
审稿时长
47 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


