6-多氟烷基-1-芳基己烷-1,3,5-三酮:合成、环链同分异构和脱水环化为 6-多氟烷基-1-芳基吡喃-4H-酮
IF 1.7
4区 化学
Q3 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
摘要
在这项工作中,我们提出了一种非对称氟烷基化三酮的合成方案。该合成基于苯甲酰基丙酮与酯在过量氢化锂存在下的反应。研究了三酮在 CDCl3 和 DMSO-d6 溶液中的同分异构平衡。证明了 1,3,5-三酮的合成应用。开发了一种通过脱水环化高效合成 2-芳基-6-(多氟烷基)-4H-吡喃-4-酮的方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
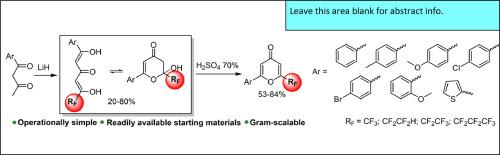
6-Polyfluoroalkyl-1-arylhexane-1,3,5-triones: Syntheses, ring-chain tautomerism and dehydrative cyclization to 6-polyfluoroalkyl-1-arylpyran-4H-ones
In this work, we present a synthetic protocol for the synthesis of non-symmetrical fluoroalkylated triketones. The synthesis is based on the reaction of benzoyl acetones with esters in the presence of excess lithium hydride. The tautomeric equilibrium of triketones in CDCl3 and DMSO‑d6 solutions was studied. The synthetic application of 1,3,5-triketones has been demonstrated. A method has been developed for the efficient synthesis of 2-aryl-6-(polyfluoroalkyl)-4H-pyran-4-ones by dehydrative cyclization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Fluorine Chemistry
化学-无机化学与核化学
CiteScore
3.80
自引率
10.50%
发文量
99
审稿时长
33 days
期刊介绍:
The Journal of Fluorine Chemistry contains reviews, original papers and short communications. The journal covers all aspects of pure and applied research on the chemistry as well as on the applications of fluorine, and of compounds or materials where fluorine exercises significant effects. This can include all chemistry research areas (inorganic, organic, organometallic, macromolecular and physical chemistry) but also includes papers on biological/biochemical related aspects of Fluorine chemistry as well as medicinal, agrochemical and pharmacological research. The Journal of Fluorine Chemistry also publishes environmental and industrial papers dealing with aspects of Fluorine chemistry on energy and material sciences. Preparative and physico-chemical investigations as well as theoretical, structural and mechanistic aspects are covered. The Journal, however, does not accept work of purely routine nature.
For reviews and special issues on particular topics of fluorine chemistry or from selected symposia, please contact the Regional Editors for further details.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


