不同温度下碳纳米管对 L-组氨酸的簇吸附
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
在单层团簇吸附模型的框架下,解释了水溶液在 25、35、45、55、65 和 80 °C 温度下 MKN-SWCNT-S1 CNT 对 L-组氨酸的吸附等温线。应用新方法确定了集束吸附等温线方程的平衡参数和 CNT 上的吸附剂结构,以及非线性建模方法。为了确认氨基酸在 CNTs 上的团簇吸附性质,应用了前人提出的聚类标准,并评估了团簇结构,这对 CNTs 在生物医学中的应用具有重要意义。应用所发现的吸附平衡参数确定了 L-组氨酸在 CNT 上的吸附热力学特性(吉布斯能、焓和熵的变化),并分析了吸附特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
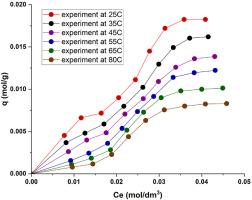
Cluster adsorption of L-histidine on carbon nanotubes at different temperatures
The adsorption isotherms of L-histidine on MKN-SWCNT-S1 CNTs from aqueous solution at temperatures of 25, 35, 45, 55, 65 and 80 °C were interpreted within the framework of the single-layer cluster adsorption model. A new approach to determine the equilibrium parameters of the cluster adsorption isotherm equation and sorbate structure on CNTs, as well as a nonlinear modelling method, was applied. To confirm the cluster nature of amino acid adsorption on CNTs, the clustering criterion proposed in the previous work was applied, and the cluster structure was evaluated, which is significant for the application of CNTs in biomedicine. The found equilibrium parameters of adsorption were applied to the determination of thermodynamic characteristics of adsorption (changes in Gibbs energy, enthalpy and entropy) of L-histidine on CNTs and the character of adsorption was analysed.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


