离子交换 LTA 沸石在吸附过程中催化 COS 的形成
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
本研究调查了改性林德 A 型(LTA)沸石在吸附处理模型天然气过程中将 H2S 和 CO2 催化反应为 COS 和 H2O 的情况。在 25 °C 的固定床吸附器中,在不同钙交换比的八种 NaA 和 CaNaA 沸石上测量了所有四种成分的突破曲线。根据交换系列材料的特性(阳离子数量、阳离子位置、阳离子类型)对 COS 突破曲线进行了讨论。COS 曲线显示,阳离子位置 II 和 III 会催化形成 COS,而位置 I 则不会形成 COS。形成的 COS 数量证明,反应水在反应终止过程中起着重要作用。反应水吸附在具有催化活性的阳离子上,因此不会再形成 COS。另一方面,进料水主要导致先前吸附的成分(CO2、H2S、COS)被置换。本文章由计算机程序翻译,如有差异,请以英文原文为准。
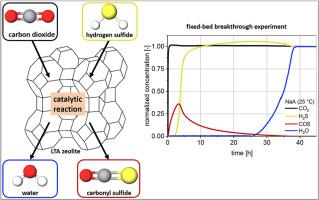
Catalytic COS formation on ion-exchanged LTA zeolites during adsorption
In this study, the catalytic reaction of H2S and CO2 to COS and H2O during the adsorptive treatment of a model natural gas on modified Linde type-A (LTA) zeolites is investigated. Breakthrough curves of all 4 components were measured at 25 °C in a fixed bed adsorber on eight NaA and CaNaA zeolites with different calcium exchange ratios. The COS breakthrough curves are discussed with regard to the properties of the materials in the exchange series (number of cations, cation position, cation type). The COS curves reveal that catalytic formation of COS occurs at cation positions II and III, whereas no COS formation occurs at position I. Ca2+-ions are more catalytically active than Na+-Ions due to their higher ionic charge. The quantities of COS formed prove that the reaction water plays a significant role in the termination of the reaction. The reaction water adsorbs on the catalytically active cations so that no further COS formation can occur. The feed water, on the other hand, mainly leads to a displacement of the previously adsorbed components (CO2, H2S, COS).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


