ETS-10 中的碱和碱土离子交换亲和力,用于水体锂分离
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
与目前去除锂源中常见杂质的方法相比,连续离子交换是一种更具可持续性的替代方法。在这项工作中,我们采用实验和计算(密度泛函理论,DFT)方法,研究了微孔钛硅酸盐 ETS-10 这种性能良好的离子交换固体与 Mg2+ 和 Ca2+ 的离子吸附相互作用。根据测量到的平衡等温线量化了使用 Na+ 形式 ETS-10 的 Mg2+ 和 Ca2+ 的离子交换亲和力,并使用修正的 Langmuir 等温线进行了分析,其中考虑到了阳离子交换过程中的整体化学计量解吸/吸附。使用 Na-ETS-10 进行离子交换的平衡常数对 Ca 的影响最大,对 Mg、K 和 Li 的影响依次减小。离子交换亲和力的这些差异与 DFT 导出的离子交换能的趋势是一致的,DFT 导出的离子交换能利用热化学循环解释了阳离子的水合和溶解。这些平衡常数值和离子交换能表明,使用 Na-ETS-10 交换 Ca2+、Mg2+ 和 K+ 比交换 Li+ 更有利。事实上,Li+ 和 K+、Mg2+ 或 Ca2+ 的等摩尔水混合物的竞争性离子交换表明,非锂阳离子会被选择性地吸收到固体中,从而在去除杂质阳离子的同时将 Li+ 浓缩到水溶液中。本文章由计算机程序翻译,如有差异,请以英文原文为准。
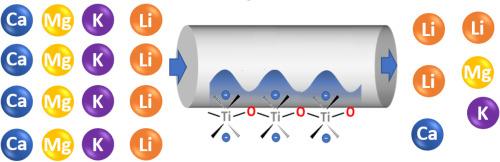
Alkali and alkaline earth ion exchange affinity in ETS-10 toward aqueous lithium separation
Continuous ion exchange is a more sustainable alternative to current methods for removing common impurities from lithium sources. In this work, we examine ion-adsorbent interactions for Mg2+ and Ca2+ with microporous titanosilicate ETS-10, an ion exchange solid with promising performance, using experimental and computational (density functional theory, DFT) methods. Ion exchange affinity for Mg2+ and Ca2+ using the Na+-form of ETS-10 are quantified from measured equilibrium isotherms, analyzed using a modified Langmuir isotherm accounting for overall stoichiometric desorption/adsorption in the cation exchange process. The equilibrium constant for ion exchange using Na-ETS-10 is greatest for Ca and decreases in order of Mg, K, and Li, respectively. These differences in ion exchange affinity are consistent with trends in DFT-derived ion exchange energies, which account for hydration and solvation of cations using a thermochemical cycle. These equilibrium constant values and ion exchange energies suggest that exchange of Ca2+, Mg2+, and K+ using Na-ETS-10 is more favorable than that of Li+. Indeed, competitive ion exchange of equimolar aqueous mixtures of Li+ and each of K+, Mg2+, or Ca2+ demonstrate selective uptake of the non-lithium cation into the solid, thereby concentrating Li+ in the aqueous solution while removing impurity cations.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


