用于头孢他啶持续给药的不含防腐剂的电纺纳米纤维插入物;设计、表征和兔眼药代动力学研究
IF 5.2
2区 医学
Q1 PHARMACOLOGY & PHARMACY
引用次数: 0
摘要
由于各种解剖和生理障碍,以及传统眼用制剂的局限性(包括生物利用度低,需要频繁给药等),眼用给药面临着巨大挑战。本文的目的是设计载入头孢他啶(CAZ)的缓释纳米纤维插件,用于治疗眼部感染,头孢他啶是一种抗生素,对革兰氏阴性和革兰氏阳性微生物有效。这些纳米纤维是利用聚乙烯醇(PVA)、聚己内酯(PCL)和 Eudragit® (EUD)等生物可降解聚合物,通过电纺丝技术制成的。纳米纤维插入物的平均直径为 250 nm,具有足够的机械强度,适合眼部使用。细胞活力测试证实了纳米纤维的无毒性和安全性。体内研究表明,纳米纤维插入物可使药物浓度超过铜绿假单胞菌和金黄色葡萄球菌的最低抑菌浓度(MIC),分别可维持 4 天和 5 天。CAZ-PVA-PCL 的 AUC0-120 为 11,882.81 ± 80.5 μg-h/ml,CAZ-PVA-EUD 的 AUC0-120 为 9649.39 ± 86.84 μg-h/ml。纳米纤维插件的药物释放时间延长,可保持有效的抗菌浓度,避免眼药水的波动,而且不含防腐剂,消除了细胞毒性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
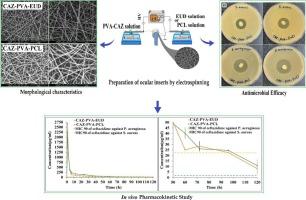
Preservative-free electrospun nanofibrous inserts for sustained delivery of ceftazidime; design, characterization and pharmacokinetic investigation in rabbit's eye
Ocular drug delivery presents significant challenges, attributed to the various anatomical and physiological barriers, as well as the limitations associated with conventional ocular formulations including low bioavailability, necessitating frequent dosing. The objective of the essay was to design sustained release nanofibrous inserts loaded with ceftazidime (CAZ), an antibiotic effective against gram-negative and gram-positive microorganisms, for the treatment of ocular infections. These nanofibers were fabricated using the electrospinning technique, employing biodegradable polymers such as polyvinyl alcohol (PVA), polycaprolactone (PCL) and Eudragit® (EUD). The nanofibrous inserts exhibited adequate mechanical strength for ocular use with an average diameter < 250 nm. In the initial 12-h period, a burst drug release was observed, followed by a controlled release for 120 h. Cell viability test confirmed the non-toxicity and safety of the nanofibers. The in vivo study demonstrated that the inserts sustain a drug concentration exceeding the minimum inhibitory concentration (MIC) of Pseudomonas aeruginosa and Staphylococcus aureus for 4 and 5 days, respectively. The AUC0–120 for CAZ-PVA-PCL was reported 11,882.81 ± 80.5 μg·h/ml and for CAZ-PVA-EUD was 9649.39 ± 86.84 μg·h/ml. The nanofibrous inserts' extended drug release maintains effective antimicrobial concentrations, avoids the fluctuations of eye drops, and, by being preservative-free, eliminates cytotoxicity.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Pharmaceutics: X
Pharmacology, Toxicology and Pharmaceutics-Pharmaceutical Science
CiteScore
6.60
自引率
0.00%
发文量
32
审稿时长
24 days
期刊介绍:
International Journal of Pharmaceutics: X offers authors with high-quality research who want to publish in a gold open access journal the opportunity to make their work immediately, permanently, and freely accessible.
International Journal of Pharmaceutics: X authors will pay an article publishing charge (APC), have a choice of license options, and retain copyright. Please check the APC here. The journal is indexed in SCOPUS, PUBMED, PMC and DOAJ.
The International Journal of Pharmaceutics is the second most cited journal in the "Pharmacy & Pharmacology" category out of 358 journals, being the true home for pharmaceutical scientists concerned with the physical, chemical and biological properties of devices and delivery systems for drugs, vaccines and biologicals, including their design, manufacture and evaluation. This includes evaluation of the properties of drugs, excipients such as surfactants and polymers and novel materials. The journal has special sections on pharmaceutical nanotechnology and personalized medicines, and publishes research papers, reviews, commentaries and letters to the editor as well as special issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


