一种基于羟乙基脲的抗冷冻水凝胶电解质,用于无树枝状晶粒的锌离子电池
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
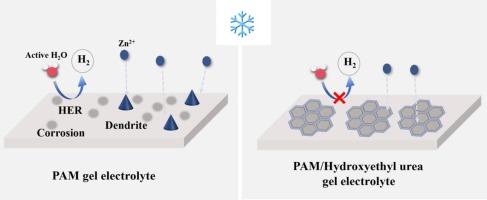
锌离子水电池因其安全性高、资源丰富和价格低廉而被视为一种前景广阔的储能资源。然而,由于枝晶的强制生成、严重的副反应和较差的温度适应性,锌离子水电池的发展一直受到严重阻碍。在此,我们采用羟乙基脲作为水凝胶电解质添加剂来解决上述难题。羟乙基脲可以破坏水的氢键(HBs),增强电解质的耐冻能力。同时,羟乙基脲还能防止锌枝晶的腐蚀问题和抑制作用。因此,Zn//Zn 对称电池在 1 mA cm-2 的条件下可持续稳定循环 3000 小时以上,库仑效率高达 99.6%。即使在零下 40 ℃ 的环境中,电池也能表现出卓越的循环稳定性,锌//锌对称电池可实现 3000 小时以上的稳定循环。它不仅在室温下表现出卓越的循环稳定性,而且在零下 40 ℃ 也能保持令人称道的功能,从而验证了该方法的有效性。这项工作为提高锌离子电池的低温性能提供了一种简单的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
An anti-freezing hydrogel electrolyte based on hydroxyethyl urea for dendrite-free Zn ion batteries
Aqueous zinc ion batteries are considered as a promising energy storage resource due to their high security, abundant resources and low price. However, the development of aqueous zinc ion batteries has been severely hindered by compulsive dendrite generation, serious side reactions and poor temperature adaptability. Herein, we used hydroxyethyl urea as a hydrogel electrolyte additive to address above-mentioned challenges. Hydroxyethyl urea can break the hydrogen bonds (HBs) of water and enhancing the freezing-tolerance ability of the electrolyte. Meanwhile, hydroxyethyl urea can prevent the corrosion issue and inhibition of Zn dendrites. Consequently, the Zn//Zn symmetric battery can sustain stable cycling for over 3000 h at 1 mA cm−2, and it achieves a high coulombic efficiency of 99.6 %. Even at −40 ℃ the batteries show excellent cycling stability, the Zn//Zn symmetry battery can achieve steadily cycles over 3000 h. The Zn//NVO battery equipped with the altered electrolyte exhibits enhanced capacity retention compared to the one without additives. It demonstrates not only excellent cycling stability at room temperature but also maintains commendable functionality down to −40 ℃, validating the method’s efficacy. This work provides a simple strategy for enhancing low temperature performance of zinc ion batteries.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


