协调铜负载纳米反应器,用于同时诱导杯状突变和干预结直肠癌的免疫治疗
IF 10.2
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
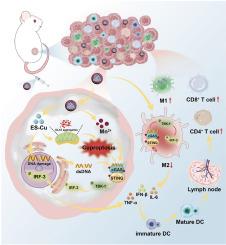
离子干扰,包括细胞内铜(Cu)超载,会破坏细胞的平衡,引发线粒体功能障碍,并激活细胞特异性死亡通道,凸显了其在癌症治疗中的巨大潜力。然而,现有的铜离子载体都是血液半衰期较短的小分子,它们在细胞内转运的铜离子不足,不可避免地影响了铜中毒的效果。在此,我们制作了ESCu@HM纳米反应器,该反应器由H-MnO2纳米颗粒与铜离子载体伊利司莫尔(ES)和铜结合自组装而成,可促进杯突效应并进一步诱导相关免疫反应。具体来说,全身循环和肿瘤积聚的 Cu 会引起不可逆的杯突症,与 Mn2+ 共同作用,导致肿瘤相关巨噬细胞(TAMs)的再极化,并放大细胞核和线粒体中受损 DNA 片段对 cGAS-STING 通路的激活。这进一步刺激了抗肿瘤免疫,并最终重编程肿瘤微环境(TME),抑制肿瘤生长。总之,ESCu@HM 作为杯突酶和免疫疗法的纳米反应器,不仅改善了 ES 的双重抗肿瘤机制,为其临床应用提供了潜在的优化方案,而且为杯突酶介导的结直肠癌(CRC)治疗的创新策略铺平了道路。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Orchestrated copper-loaded nanoreactor for simultaneous induction of cuproptosis and immunotherapeutic intervention in colorectal cancer
Ion interference, including intracellular copper (Cu) overload, disrupts cellular homeostasis, triggers mitochondrial dysfunction, and activates cell-specific death channels, highlighting its significant potential in cancer therapy. Nevertheless, the insufficient intracellular Cu ions transported by existing Cu ionophores, which are small molecules with short blood half-lives, inevitably hamper the effectiveness of cuproptosis. Herein, the ESCu@HM nanoreactor, self-assembled from the integration of H-MnO2 nanoparticles with the Cu ionophore elesclomol (ES) and Cu, was fabricated to facilitate cuproptosis and further induce relevant immune responses. Specifically, the systemic circulation and tumoral accumulation of Cu, causing irreversible cuproptosis, work in conjunction with Mn2+, resulting in the repolarization of tumor-associated macrophages (TAMs) and amplification of the activation of the cGAS-STING pathway by damaged DNA fragments in the nucleus and mitochondria. This further stimulates antitumor immunity and ultimately reprograms the tumor microenvironment (TME) to inhibit tumor growth. Overall, ESCu@HM as a nanoreactor for cuproptosis and immunotherapy, not only improves the dual antitumor mechanism of ES and provides potential optimization for its clinical application, but also paves the way for innovative strategies for cuproptosis-mediated colorectal cancer (CRC) treatment.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


