新型纳米药物融合了铁跃迁和光热疗法,非常适合 PD-L1 介导的免疫检查点阻断疗法
IF 8.7
1区 医学
Q1 ENGINEERING, BIOMEDICAL
引用次数: 0
摘要
基于免疫检查点阻断的免疫疗法已成为一种前景广阔的治疗策略;然而,其疗效受到免疫抑制微环境的限制。在这里,我们开发了一种新型免疫激活纳米粒子(Fc-SS-Fe/Cu)来解决免疫渗透不足的问题。具体来说,Fc-SS-Fe/Cu的结构会随着肿瘤微环境的变化而发生塌缩,二茂铁和二硫键以及释放的Fe/Cu离子能有效生成-OH并消耗GSH,从而增加氧化应激,进而诱导铁变态反应。最终,"激光触发的温和温度光热疗法(PTT)、PTT 加速铁跃迁和 LPO 积累、LPO 介导的 HSPs 下调促进 PTT "的正反馈机制有效地引发了肿瘤细胞的免疫原性细胞死亡(ICD),显著增强了肿瘤细胞的免疫原性。此外,Fc-SS-Fe/Cu诱导的O2生成能力可逆转缺氧的肿瘤微环境,重要的是,通过抑制HIF-1α通路,可有效下调肿瘤细胞表面PD-L1的表达,从而增强抗PD-L1(αPD-L1)治疗的效果。因此,本研究为加强 PD-L1 介导的 ICB 治疗提供了有价值的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
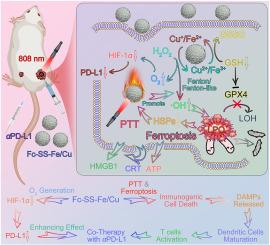
A novel nanomedicine integrating ferroptosis and photothermal therapy, well-suitable for PD-L1-mediated immune checkpoint blockade
Immunotherapy based on immune checkpoint blockade has emerged as a promising treatment strategy; however, the therapeutic efficacy is limited by the immunosuppressive microenvironment. Here, we developed a novel immune-activated nanoparticle (Fc-SS-Fe/Cu) to address the issue of insufficient immune infiltration. Specifically, the structure of Fc-SS-Fe/Cu collapsed in response to the tumor microenvironment, the ferrocene and disulfide bonds and the released Fe/Cu ions can effectively generate ·OH and deplete GSH to increase oxidative stress, thereby inducing ferroptosis. Withal, the positive feedback mechanisms of "laser-triggered mild-temperature photothermal therapy (PTT), PTT accelerated ferroptosis and LPO accumulation, LPO mediated HSPs down-regulated to promote PTT," effectively triggers immunogenic cell death (ICD) in tumor cells, significantly enhancing their immunogenicity. Moreover, the O2-generating ability induced by Fc-SS-Fe/Cu could reverse the hypoxic tumor microenvironment, and importantly, the expression of PD-L1 on tumor cell surfaces could be effectively downregulated by inhibiting the HIF-1α pathways, thereby augmenting the effect of anti-PD-L1 (αPD-L1) therapy. Therefore, this study provides valuable strategies into enhancing PD-L1-mediated ICB therapy.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Materials Today Bio
Multiple-
CiteScore
8.30
自引率
4.90%
发文量
303
审稿时长
30 days
期刊介绍:
Materials Today Bio is a multidisciplinary journal that specializes in the intersection between biology and materials science, chemistry, physics, engineering, and medicine. It covers various aspects such as the design and assembly of new structures, their interaction with biological systems, functionalization, bioimaging, therapies, and diagnostics in healthcare. The journal aims to showcase the most significant advancements and discoveries in this field. As part of the Materials Today family, Materials Today Bio provides rigorous peer review, quick decision-making, and high visibility for authors. It is indexed in Scopus, PubMed Central, Emerging Sources, Citation Index (ESCI), and Directory of Open Access Journals (DOAJ).
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


