一种原甲酸酯桥接的双偶氮二元醇:生理条件下增强的一氧化氮负荷和释放的一氧化氮
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
在生物医学应用中,二氮杂环戊烷因其卓越的氮氧化物负载效率而被高度评价为氮氧化物供体。通常情况下,这些化合物的氮氧化物释放特性可以通过在 N-二氮杂环戊烯的 O2 位加入各种替代物来定制。在本研究中,我们报告了一类新型的双二偶氮二酸盐,它们通过两个二偶氮二酸盐基团之间的原甲酸桥共价连接。在生理条件下(pH = 7.4,37 °C),这些原甲酸桥双偶氮二酸盐可释放四当量的 NO。释放 NO 的半衰期在 1.3 到 17.9 分钟之间。此外,与商业化的 2-(N,N-二乙基氨基)-diazenolate-2-氧化物相比,这些双二苯二酚对大肠杆菌(MIC = 1-8 mM,MBC = 8-32 mM)和金黄色葡萄球菌(MIC = 2-8 mM,MBC = 16-32 mM)具有更强的杀菌活性。大肠杆菌)= 32 mM,MIC(金黄色葡萄球菌)= 64 mM,MBC=128 mM)。这些双二偶氮二氧化物能提高氮氧化物的负载量并释放氮氧化物,而不依赖于酶或化学添加剂,这表明它们具有进一步应用于生物医学的潜在优势。本文章由计算机程序翻译,如有差异,请以英文原文为准。
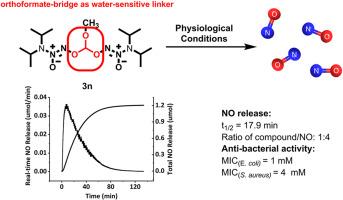
An orthoformate-bridged bis-diazeniumdiolates: Enhanced nitric oxide loading and released NO under physiological conditions
Diazeniumdiolates are highly valued as NO donors in biomedical applications because of their exceptional NO-loading efficiency. Typically, the NO-releasing properties of these compounds can be tailored by incorporating various substitutes at the O2-position of N-diazeniumdiolates. In this study, we report a novel class of bis-diazeniumdiolates, which are covalently linked through an orthoformate-bridge between two diazeniumdiolate groups. Under physiological conditions (pH = 7.4, 37 °C), these orthoformate-bridged bis-diazeniumdiolates were found to release four equivalents of NO. The half-lives of NO release varied between 1.3 and 17.9 min. Furthermore, these bis-diazeniumdiolates exhibited superior bactericidal activity against Escherichia coli (MIC = 1–8 mM, MBC = 8–32 mM) and Staphylococcus aureus (MIC = 2–8 mM, MBC = 16–32 mM) compared to the commercial 2-(N, N-diethylamino)-diazenolate-2-oxide, which only releases two equivalents of NO per molecular (MIC (E. coli) = 32 mM, MIC (S.aureus) = 64 mM, MBC = 128 mM). These bis-diazeniumdiolates enhance NO loading and release NO independently of enzymes or chemical additives, indicating their potential benefits for further biomedical applications.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


