Ca(II) 与苯丙二酸的新型金属有机框架的光催化和传感研究
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
利用凝胶扩散法制备了一种新的苯丙二酸 Ca(II) 金属有机框架 [Ca(PMA)]n。单晶 X 射线衍射数据显示,该晶体属于单斜空间群 C2/c。在晶体结构中,苯基丙二酸配体与 Ca(II)离子采用双单齿配位和双齿配位模式。苯基丙二酸钙配体单元的三维延伸形成了网络结构。热重研究证实,[Ca(PMA)]n 的晶体结构在 160 ℃ 以下都很稳定。在阳光下对亚甲基蓝进行的光催化染料降解研究表明,降解效率为 60.8%。当标题化合物在 260 纳米波长处被激发时,可在 290 纳米波长处观察到强烈的发光发射。此外,还研究了[Ca(PMA)]n 与 Fe3+ 离子的传感特性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
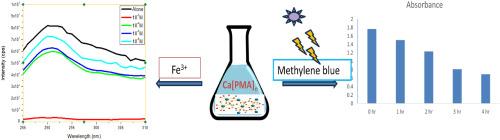
Photocatalytic and sensing studies of a new metal-organic framework of Ca(II) with phenylmalonic acid
A new metal-organic framework of Ca(II) with phenylmalonic acid, [Ca(PMA)]n has been prepared by gel diffusion method. Single crystal X-ray diffraction data show that the crystal belongs to the monoclinic space group C2/c. In the crystal structure, the phenylmalonate ligand adopts bis-monodentate and bidentate coordination modes with the Ca(II) ions. The Ca-phenylmalonate units extend three dimensionally forming a network structure. Thermogravimetric studies confirm that the crystal structure of [Ca(PMA)]n is stable up to 160 °C. Photocatalytic dye degradation studies on methylene blue under sunlight shows 60.8 % degradation efficiency. When the title compound is excited at 260 nm, strong luminescence emission at 290 nm was observed. Sensing property of [Ca(PMA)]n with Fe3+ ions was also investigated.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


