温和条件下涉及香豆素-3-甲酰基吡唑的意想不到的迈克尔式加成-去酰基吡唑
IF 2.1
3区 化学
Q2 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用 DMAP 作为催化剂,在温和条件下首次完成了涉及 α、β-不饱和吡唑酰胺的迈克尔加成-去乙酰基吡唑。该方法可以从香豆素-3-甲酰基吡唑和 3-乙酰氧基氧茚酮中获得各种吲哚取代的二氢香豆素,收率极高(高达 99%),非对映选择性也可以接受(高达 95:5dr)。在这项工作中,香豆素-3-甲酰基吡唑的酰基吡唑基团首次充当了临时活化基团。通过高分辨率质谱分析进行了详细的机理研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
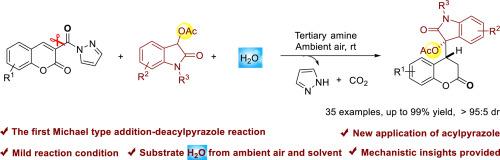
An unexpected Michael type addition-deacylpyrazole involving coumarin-3-formylpyrazoles under mild conditions
The first Michael type addition-deacylpyrazole involving α,β-unsaturated pyrazolamides was accomplished by utilizing DMAP as catalyst in mild condition. The protocol enables the access to various oxindole-substituted dihydrocoumarins from coumarin-3-formylpyrazoles and 3-acetoxy oxindoles in excellent yields (up to 99 % yield) with acceptable diastereoselectivities (up to >95:5 dr). In this work, the acylpyrazole group of coumarin-3-formylpyrazoles acted as a temporary activating group for the first time. Detailed mechanistic studies were performed with high resolution mass spectrometry (HRMS) analysis.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron
化学-有机化学
CiteScore
3.90
自引率
4.80%
发文量
439
审稿时长
34 days
期刊介绍:
Tetrahedron publishes full accounts of research having outstanding significance in the broad field of organic chemistry and its related disciplines, such as organic materials and bio-organic chemistry.
Regular papers in Tetrahedron are expected to represent detailed accounts of an original study having substantially greater scope and details than that found in a communication, as published in Tetrahedron Letters.
Tetrahedron also publishes thematic collections of papers as special issues and ''Reports'', commissioned in-depth reviews providing a comprehensive overview of a research area.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


