精确预测包晶 XPH3(X=Li、Na、K)氢化物的储氢能力:第一原理研究
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
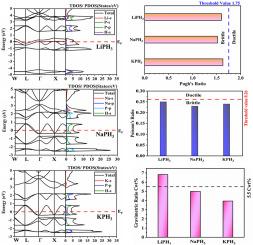
氢储存仍然是创造可持续氢经济的一个重大障碍,因为目前的许多材料都无法满足对安全性、效率和容量的高要求。目前的储氢技术往往表现出较低的重力密度和较慢的吸收/解吸速率,这限制了它们在能源系统中的实际应用。本手稿报告了碱基包晶氢化物 LiPH3、NaPH3 和 KPH3 的物理特性及其储氢潜力的第一性原理分析。体积优化曲线、负形成焓和容限因子表明了所研究的这些氢化物具有完全的结构和几何稳定性。LiPH3、NaPH3 和 KPH3 的机械属性显示了脆性、较高的抗压缩性、耐高温性和各向异性。在晶体平面上观察到较高的纵向速度。XPH3(X = Li、Na、K)的方向速度反映了每个晶面的各向异性。电子能带结构、TDOS 和 PDOS 阐明了所研究的这些氢化物的金属特性。这些氢化物的光学特性表明,它们在紫外光谱中具有良好的光学传导性,同时在紫外区的极化和色散程度极小。LiPH3 (6.83 wt%)、NaPH3 (5.00 wt%) 和 KPH3 (3.95 wt%) 的储氢能力表明,所有包晶体氢化物在储氢方面都取得了可喜的成果,但 LiPH3 是储氢方面最有力的竞争者,具有最高的重量比(6.83 wt%)和体积储氢能力(93.39 gH2/L),因为它满足了美国能源部提到的 2025 年金属氢化物的储能需求。本文章由计算机程序翻译,如有差异,请以英文原文为准。
A precise prediction for the hydrogen storage ability of perovskite XPH3 (X=Li, Na, K) hydrides: First-principles study
Hydrogen storage remains a significant barrier to creating a sustainable hydrogen economy, as many current materials fail to meet the high safety, efficiency, and capacity requirements. Current hydrogen storage technologies frequently exhibit low gravimetric densities and slow absorption/desorption rates, which limit their practical applicability in energy systems. This manuscript reports the first principles analysis on the physical features of alkali-based perovskite hydrides LiPH3, NaPH3, and KPH3, along with their hydrogen storage potential. Volume optimization curves, negative formation enthalpies and tolerance factor manifested the complete structural and geometric stability of these studied hydrides. Brittle, higher resistance to indentation, endurance towards high temperatures and anisotropic behavior are revealed through mechanical attributes for LiPH3, NaPH3, and KPH3. Higher longitudinal velocities are observed in crystallographic planes. The directional velocities for XPH3 (X = Li, Na, K) reflect an anisotropic nature in each crystallographic plane. The electronic band structure, TDOS and PDOS elaborates the metallic behavior of these studied hydrides. These hydrides' optical characteristics showed that they have good optical conductivity in the UV spectrum, along with minimal polarization and dispersion in the UV region. The hydrogen storage capacities for LiPH3 (6.83 wt%), NaPH3 (5.00 wt%), and KPH3 (3.95 wt%) signifies that all perovskite hydrides have shown promising results for hydrogen storage but LiPH3 is the strongest contender for hydrogen storage with highest gravimetric ratio (6.83 wt%) and volumetric storage (93.39 gH2/L) as it fulfills the energy storage demand mentioned by US-DOE of metal hydrides for year 2025.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


