将 La0.6Sr0.4FeO3 -δ 颗粒作为化学循环应用中的氧气载体的实验研究
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
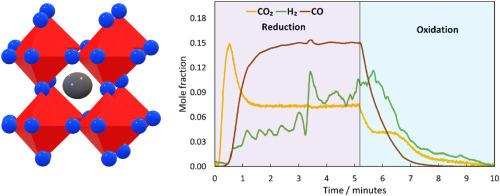
镧锶铁氧体(LSF)作为氧气载体,可用于化学循环法生产 H2 和利用 CO2。在这项工作中,我们首次尝试在动态运行的填料床反应器中使用 400 克 LSF 粒子来放大和了解传热和传质情况。实验在 650-820 °C 和 1-5 barg 条件下进行,评估了颗粒在化学环路水气变换(CL-WGS)和化学环路反向水气变换(CL-RWGS)反应的高温氧化还原循环中 70 小时的反应器热管理、相稳定性和机械完整性。对放热和内热氧化还原反应的反应器热量管理表明,在 15 厘米反应床长度上,热量前沿滞后于反应前沿的情况下,能够保持较高的温度曲线。在 LSF 床层与 H2O 和 CO2 的氧化过程中,观察到的最大 ΔT 分别为 45 °C 和 35 °C。在空气氧化的情况下,最大 ΔT 为 120 °C,这表明反应的放热程度较高,尤其是在需要热量来维持反应过程时,可以用来提高反应床的温度。在 70 小时的测试过程中,没有记录到材料性能退化的迹象,保持了颗粒的可循环操作性、相稳定性和机械完整性。这些结果表明了这种材料的坚固性,而且对于 LSF 在化学循环应用、H2 生产和 CO2 利用过程中的可扩展性来说,也是令人鼓舞的。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Experimental investigation of La0.6Sr0.4FeO3 -δ pellets as oxygen carriers for chemical-looping applications
Lanthanum Strontium Ferrite (LSF), as an oxygen carrier, is used for chemical looping H2 production and CO2 utilization. In this work, the first attempt to upscale and understand the heat and mass transfer using 400 g of LSF pellets in a dynamically operated packed bed reactor is carried out.
Experiments were conducted at 650–820 °C and 1–5 barg, evaluating the pellets' reactor heat management, phase stability, and mechanical integrity over 70 h for the high-temperature redox cycling in chemical looping water-gas shift (CL-WGS) and chemical looping reverse water-gas shift (CL-RWGS) reactions.
Key results at the reactor scale indicate a maximum cyclic average H2O-to-H2 conversion of 31%, with a peak oxygen carrier capacity for CL-WGS of 0.38 mmol/gLSF. In the case of CL-RWGS, a peak CO2-to-CO conversion of 99.8% was achieved, with an oxygen carrier capacity of 0.57 mmol/gLSF.
Reactor heat management for exothermic and endothermic redox reactions showed the ability to maintain a high temperature profile where the heat front lagged the reaction front over a 15 cm reactive bed length. A maximum ΔT of 45 °C and 35 °C were observed during the oxidation of the LSF bed with H2O and CO2, respectively. In the case of air oxidation, a maximum ΔT of 120 °C indicates that the reaction is more exothermic and can be used to raise the temperature of the bed especially if heat is required to sustain the process.
No evidence of material performance degradation was recorded over 70 h of testing, maintaining the pellets' operational cyclability, phase stability, and mechanical integrity. The results demonstrate the robustness of the material, and they are encouraging versus the scalability of LSF for chemical looping applications, into H2 production and CO2 utilization processes.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


