用于高温应用(如固体氧化物电池堆中的互连器件)的 Crofer 22 H 铁素体钢在空气和 Ar-H2-H2O 大气中形成的氧化鳞片的氧化动力学和电气特性
IF 8.3
2区 工程技术
Q1 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
在空气和 Ar-H2-H2O 混合气体(p(H2)/p(H2O) = 94/6)中,在 973-1123 K 的范围内对 Crofer 22H 进行了 100 小时等温氧化动力学研究。在 1023 和 1073 K 时,抛物线速率常数在 6.2 × 10-24 到 0.21 atm 的范围内与氧分压无关,而在 973 和 1123 K 时,空气中的速率常数高于 Ar-H2-H2O 中的速率常数。鳞片由 Cr2O3 和锰铬尖晶石组成,锰铬比取决于氧化条件。采用 X 射线衍射、扫描电子显微镜、共焦拉曼成像和特定区域电阻测量等多种方法,对这种钢在固体氧化物燃料电池中的应用进行了跨尺度电阻评估。本文章由计算机程序翻译,如有差异,请以英文原文为准。
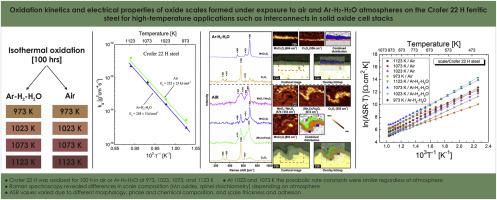
Oxidation kinetics and electrical properties of oxide scales formed under exposure to air and Ar–H2-H2O atmospheres on the Crofer 22 H ferritic steel for high-temperature applications such as interconnects in solid oxide cell stacks
A 100 h isothermal oxidation kinetics study for Crofer 22H was conducted in air and the Ar–H2-H2O gas mixture (p(H2)/p(H2O) = 94/6) in the range of 973–1123 K. The parabolic rate constant was independent of oxygen partial pressure in the range from 6.2 × 10−24 to 0.21 atm at 1023 and 1073 K, while at 973 and 1123 K it was higher in air than in Ar–H2-H2O. The scales consisted of Cr2O3 and manganese chromium spinel with an Mn:Cr ratio dependent on the oxidation conditions. Cross-scale resistance was evaluated with regards to the application of the steel in solid oxide fuel cells using a number of methods, including X-ray diffraction, scanning electron microscopy, confocal Raman imaging and area-specific resistance measurements.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

International Journal of Hydrogen Energy
工程技术-环境科学
CiteScore
13.50
自引率
25.00%
发文量
3502
审稿时长
60 days
期刊介绍:
The objective of the International Journal of Hydrogen Energy is to facilitate the exchange of new ideas, technological advancements, and research findings in the field of Hydrogen Energy among scientists and engineers worldwide. This journal showcases original research, both analytical and experimental, covering various aspects of Hydrogen Energy. These include production, storage, transmission, utilization, enabling technologies, environmental impact, economic considerations, and global perspectives on hydrogen and its carriers such as NH3, CH4, alcohols, etc.
The utilization aspect encompasses various methods such as thermochemical (combustion), photochemical, electrochemical (fuel cells), and nuclear conversion of hydrogen, hydrogen isotopes, and hydrogen carriers into thermal, mechanical, and electrical energies. The applications of these energies can be found in transportation (including aerospace), industrial, commercial, and residential sectors.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


