代谢组图谱区分门静脉血管疾病、肝硬化和健康人
IF 9.5
1区 医学
Q1 GASTROENTEROLOGY & HEPATOLOGY
引用次数: 0
摘要
背景& 目的门静脉血管病变(PSVD)是一种罕见的血管性肝病,在诊断上具有挑战性。本研究旨在确定PSVD或肝硬化患者独特的代谢组学特征,以促进无创诊断并阐明受干扰的代谢途径。方法分析了20名健康志愿者(HVs)、20名经组织学证实的PSVD患者或20名肝硬化患者的血清样本。代谢物采用液相色谱-质谱法进行测定。差异丰度用 Limma 修正的 t 统计量进行评估。研究人员开发了人工神经网络和支持向量机模型,将 PSVD 与肝硬化或 HV 代谢组学特征进行分类。结果 共有283个代谢物被纳入下游分析。聚类分析有效地将 PSVD 与 HV 代谢组分开,尽管 PSVD 患者(5 人,25%)与肝硬化患者重叠。差异检验发现了与 PSVD 相关的重大代谢紊乱,包括牛磺胆酸和脂肪酸的中枢变化,从而将 PSVD 患者与 HV 和肝硬化患者区分开来。嘧啶、甘氨酸、丝氨酸和苏氨酸通路的改变与 PSVD 完全相关。利用精选代谢特征的机器学习模型只需使用 4 到 6 个代谢物就能可靠地将 PSVD 组与 HV 或肝硬化患者区分开来。在一个独立队列中进行的验证表明,牛磺胆酸(AUROC 0.899)对 PSVD 患者与 HVs 具有很高的区分能力,牛磺胆酸/天冬氨酸比值(AUROC 0.720)对 PSVD 与肝硬化具有很高的区分能力。影响和意义:门静脉血管病变(PSVD)是一种血管性肝病,可在无肝硬化的情况下导致门静脉前高压,其病理生理学尚不清楚,也没有成熟的治疗方法。我们的研究表明,分析血清代谢组可以发现 PSVD 患者的不同代谢特征,包括嘧啶、甘氨酸、丝氨酸和苏氨酸通路的改变,从而揭示该疾病的潜在通路。这些发现可以让人们更早地对 PSVD 进行非侵入性诊断,从而减少对肝活检等侵入性程序的依赖,并为诊断路径提供指导。本文章由计算机程序翻译,如有差异,请以英文原文为准。
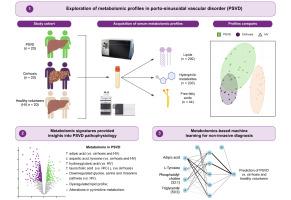
Metabolomic profiles differentiate between porto-sinusoidal vascular disorder, cirrhosis, and healthy individuals
Background & Aims
Porto-sinusoidal vascular disorder (PSVD) is a rare and diagnostically challenging vascular liver disease. This study aimed to identify distinct metabolomic signatures in patients with PSVD or cirrhosis to facilitate non-invasive diagnosis and elucidate perturbed metabolic pathways.
Methods
Serum samples from 20 healthy volunteers (HVs), 20 patients with histologically confirmed PSVD or 20 patients with cirrhosis were analyzed. Metabolites were measured using liquid chromatography-mass spectrometry. Differential abundance was evaluated with Limma’s moderated t-statistics. Artificial neural network and support vector machine models were developed to classify PSVD against cirrhosis or HV metabolomic profiles. An independent cohort was used for validation.
Results
A total of 283 metabolites were included for downstream analysis. Clustering effectively separated PSVD from HV metabolomes, although a subset of patients with PSVD (n = 5, 25%) overlapped with those with cirrhosis. Differential testing revealed significant PSVD-linked metabolic perturbations, including pertubations in taurocholic and adipic acids, distinguishing patients with PSVD from both HVs and those with cirrhosis. Alterations in pyrimidine, glycine, serine, and threonine pathways were exclusively associated with PSVD. Machine learning models utilizing selected metabolic signatures reliably differentiated the PSVD group from HVs or patients with cirrhosis using only 4 to 6 metabolites. Validation in an independent cohort demonstrated the high discriminative ability of taurocholic acid (AUROC 0.899) for patients with PSVD vs. HVs and the taurocholic acid/aspartic acid ratio (AUROC 0.720) for PSVD vs. cirrhosis.
Conclusions
High-throughput metabolomics enabled the identification of distinct metabolic profiles that differentiate between PSVD, cirrhosis, and healthy individuals. Unique alterations in the glycine, serine, and threonine pathways suggest their potential involvement in PSVD pathogenesis.
Impact and implications:
Porto-sinusoidal vascular disorder (PSVD) is a vascular liver disease that can lead to pre-sinusoidal portal hypertension in the absence of cirrhosis, with poorly understood pathophysiology and no established treatment. Our study demonstrates that analyzing the serum metabolome could reveal distinct metabolic signatures in patients with PSVD, including alterations in the pyrimidine, glycine, serine and threonine pathways, potentially shedding light on the disease's underlying pathways. These findings could enable earlier and non-invasive diagnosis of PSVD, potentially reducing reliance on invasive procedures like liver biopsy and guiding diagnostic pathways.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

JHEP Reports
GASTROENTEROLOGY & HEPATOLOGY-
CiteScore
12.40
自引率
2.40%
发文量
161
审稿时长
36 days
期刊介绍:
JHEP Reports is an open access journal that is affiliated with the European Association for the Study of the Liver (EASL). It serves as a companion journal to the highly respected Journal of Hepatology.
The primary objective of JHEP Reports is to publish original papers and reviews that contribute to the advancement of knowledge in the field of liver diseases. The journal covers a wide range of topics, including basic, translational, and clinical research. It also focuses on global issues in hepatology, with particular emphasis on areas such as clinical trials, novel diagnostics, precision medicine and therapeutics, cancer research, cellular and molecular studies, artificial intelligence, microbiome research, epidemiology, and cutting-edge technologies.
In summary, JHEP Reports is dedicated to promoting scientific discoveries and innovations in liver diseases through the publication of high-quality research papers and reviews covering various aspects of hepatology.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


