通过大规范密度泛函理论揭示用于 CO2-甲醇电催化的螺环悬挂基钴酞菁
IF 4.9
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
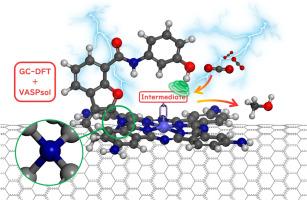
异质酞菁钴(CoPc)是电催化将二氧化碳还原为甲醇的少数几种有利分子催化剂之一。平面共轭酞菁配体的平面内取代基修饰是建立钴酞菁的结构-活性关系并提高其催化性能的关键方法。受高效酶催化结构启发而产生的立体悬挂取代基在金属卟啉体系中非常普遍,但它们在金属酞菁中的应用仍未得到充分探索。在此,我们对悬挂基团取代 CoPc 的异相电催化二氧化碳还原反应进行了系统的恒电位理论研究。值得注意的是,我们发现一种基于苯酚的悬挂基团修饰的四氨基钴酞菁在甲醇生产中具有优异的活性和选择性。有趣的是,悬挂基团取代基通过螺环结构(而不是传统的 C-N 单键)与配体连接,这可以减少空间立体阻碍并保持氢键。悬挂基团中的酚类 H 原子与 *CHO 和 *CH2O 关键结构中的 O 原子形成有效的氢键,从而产生强大的稳定作用,在较宽的电位范围内促进决定选择性的 *CO → *CHO 和决定速率的 *CHO → *CH2O。我们的发现为金属酞菁催化剂提供了一种理论上有效的悬挂基取代基,拓宽了取代基设计的范围和复杂性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Spiro-linked hanging group cobalt phthalocyanine for CO2-to-methanol electrocatalysis unveiled by grand canonical density functional theory
Heterogeneous cobalt phthalocyanine (CoPc) is one of the few favorable molecular catalysts for the electrocatalytic reduction of carbon dioxide to methanol. In-plane substituent modification of planar conjugated phthalocyanine ligands is a key way to establish the structure-activity relationship and enhance the catalytic performance of cobalt phthalocyanines. While steric hanging substituents inspired by efficient enzymes' catalytic architectures are prevalent in metalloporphyrin systems, their application in metal phthalocyanines remains underexplored. Herein, we carried out a systematic constant potential theoretical study on heterogeneous hanging group substituted CoPc electrocatalytic carbon dioxide reduction reaction. Notably, a phenol-based hanging group-modified tetra amino cobalt phthalocyanine was found to have excellent activity and selectivity for the methanol production. Intriguingly, the hanging group substituent connects to the ligand through a spirocyclic structure (rather than the conventional C-N single bond), which can reduce spatial steric hindrance and maintain hydrogen bonding. The phenolic H atoms in the hanging group form effective hydrogen bonds with the O atoms in the key structures of *CHO and *CH2O, resulting in a strong stabilizing effect that facilitates the selectivity-determining *CO → *CHO and rate-determining *CHO → *CH2O over a wide potential range. Our findings offer a theoretically effective hanging group substituent for metal phthalocyanine catalysts, broadening the scope and complexity of substituent design.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Molecular Catalysis
Chemical Engineering-Process Chemistry and Technology
CiteScore
6.90
自引率
10.90%
发文量
700
审稿时长
40 days
期刊介绍:
Molecular Catalysis publishes full papers that are original, rigorous, and scholarly contributions examining the molecular and atomic aspects of catalytic activation and reaction mechanisms. The fields covered are:
Heterogeneous catalysis including immobilized molecular catalysts
Homogeneous catalysis including organocatalysis, organometallic catalysis and biocatalysis
Photo- and electrochemistry
Theoretical aspects of catalysis analyzed by computational methods
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


