质子耦合电子传递促成的β-C-C 裂变,可见光诱导环醇与乙烯基氮杂烯烃和烯酮的开环交叉偶联
IF 9.4
1区 化学
Q1 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
吡啶酮和 1,6-二酮都是极具吸引力和价值的支架,它们在大量重要天然产物、药物、有机材料和精细化学品的合成和结构修饰中发挥着关键作用。在此背景下,我们在此展示了一种前所未有、稳健且普遍适用的合成策略,通过可见光诱导的环醇与乙烯基氮烯和烯酮的开环偶联反应,分别提供这两种关键的酮框架。其合理的机理包括:质子耦合电子转移使环醇的 β-C-C 键发生选择性裂解,随后发生 Giese 型加成反应,接着发生单电子还原和质子化反应。该合成方法具有广泛的底物范围、良好的官能团兼容性、操作简便性和环境友好性。本文章由计算机程序翻译,如有差异,请以英文原文为准。
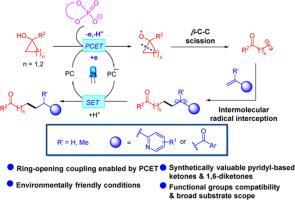
Visible-light-induced ring-opening cross-coupling of cycloalcohols with vinylazaarenes and enones via β-C-C scission enabled by proton-coupled electron transfer
Pyridyl-based ketones and 1,6-diketones are both attractive and invaluable scaffolds which play pivotal roles in the construction and structural modification of a plethora of synthetically paramount natural products, pharmaceuticals, organic materials and fine chemicals. In this context, we herein demonstrate an unprecedented, robust and generally applicable synthetically strategy to deliver these two crucial ketone frameworks via visible-light-induced ring-opening coupling reactions of cycloalcohols with vinylazaarenes and enones, respectively. A plausible mechanism involves the selective β-C-C bond cleavage of cycloalcohols enabled by proton-coupled electron transfer and ensuing Giese-type addition followed by single electron reduction and protonation. The synthetic methodology exhibits broad substrate scope, excellent functional group compatibility as well as operational simplicity and environmental friendliness.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chinese Chemical Letters
化学-化学综合
CiteScore
14.10
自引率
15.40%
发文量
8969
审稿时长
1.6 months
期刊介绍:
Chinese Chemical Letters (CCL) (ISSN 1001-8417) was founded in July 1990. The journal publishes preliminary accounts in the whole field of chemistry, including inorganic chemistry, organic chemistry, analytical chemistry, physical chemistry, polymer chemistry, applied chemistry, etc.Chinese Chemical Letters does not accept articles previously published or scheduled to be published. To verify originality, your article may be checked by the originality detection service CrossCheck.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


