3-乙氧基羰基香豆素-8-丙酰胺的合成、抗氧化和抗糖尿病潜力评估
IF 3.2
4区 化学
Q2 CHEMISTRY, MULTIDISCIPLINARY
引用次数: 0
摘要
研究人员设计、合成了一系列 3- 乙氧羰基香豆素-8-丙酰胺类似物,并通过 DPPH 和 ABTS 试验筛选了这些类似物的体外抗氧化潜力,同时考察了它们对α-淀粉酶的抗糖尿病活性。与标准品三氯氧磷(IC50 - 43.3 μM)和抗坏血酸(IC50 - 42.1 μM)相比,3-氟-2-三氟甲基苯基取代的香豆素-8-丙酰胺(8k)对 DPPH 和 ABTS 自由基具有极佳的清除潜力(IC50 - 43.1 μM)。二氰基苯取代的丙酰胺类似物(8m)对α-淀粉酶的抑制作用最强,IC50 值为 5.1 μM,与二甲双胍(IC50 4.6 μM)相当。化合物与受体蛋白α-淀粉酶(PDB ID 4x9y)的对接研究显示,与二甲双胍相比,化合物的结合能更低。本文章由计算机程序翻译,如有差异,请以英文原文为准。
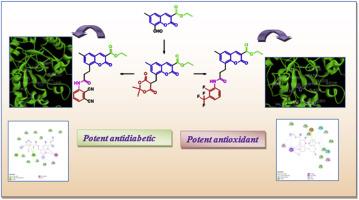
Synthesis, evaluation of antioxidant and antidiabetic potential of 3-ethoxy carbonyl coumarin-8-propionamides
A series of 3- ethoxycarbonyl coumarin-8-propionamide analogues were designed, synthesized and in vitro antioxidant potential was screened by DPPH and ABTS assays and examined their anti-diabetic activity against α-amylase enzyme. The 3-fluoro-2-trifluoromethyl phenyl substituted coumarin-8-propionamide (8k) showed excellent scavenging potential (IC50 - 43.1 μM) for both DPPH and ABTS free radicals compared with the standards trolox (IC50 - 43.3 μM) and ascorbic acid (IC50 - 42.1 μM). The compound dicyanophenyl substituted propionamide analogue (8m) displayed highest inhibition with IC50 value of 5.1 μM against the α-amylase enzyme which is well comparable with Metformin (IC50 4.6 μM). The docking studies of the compounds with the receptor protein α-amylase (PDB ID 4x9y) shown lower binding energy when compared with Metformin.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
3.50
自引率
7.70%
发文量
492
审稿时长
3-8 weeks
期刊介绍:
The Journal of the Indian Chemical Society publishes original, fundamental, theorical, experimental research work of highest quality in all areas of chemistry, biochemistry, medicinal chemistry, electrochemistry, agrochemistry, chemical engineering and technology, food chemistry, environmental chemistry, etc.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


