通过酸催化分子内 Diels-Alder 环加成正式合成阿魏精醇甲醚
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
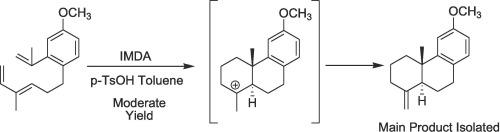
芳香族双乙烷二萜以其广泛的生物和医药活性而闻名,引起了合成化学家和药物化学家的极大兴趣。虽然一些芳香族双乙烷二萜在自然界中含量丰富,但许多芳香族双乙烷二萜的数量有限,这限制了它们在详细生物学研究中的应用。我们启动了一个合成这些化合物的项目,作为我们的第一种方法,我们描述了铁杉醇甲醚的正式合成。从 Tiglic 醛(6)和 2-溴-5-甲氧基苯甲酸(11)开始,我们通过九个步骤合成了三烯(3)。关键的转化过程是在酸催化下对三烯(3)进行分子内 Diels-Alder (IMDA) 环化,生成 12-甲氧基-19-去甲二碳-4(18),8,11,13-四烯(14)作为主要产物,十个步骤的总收率为 17.3%。虽然收率不高,但这种方法为合成阿比坦类化合物提供了一条新途径。我们正在积极探索提高总产率和开发不对称合成变体的策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Formal synthesis of ferruginol methyl ether via an acid-catalysed intramolecular Diels–Alder cycloaddition
Aromatic abietane diterpenoids, known for their wide range of biological and pharmaceutical activities, have attracted considerable interest from synthetic and medicinal chemists. Although some aromatic abietane diterpenoids are naturally abundant, many are only available in limited quantities, constraining their use in detailed biological investigations. We initiated a project to synthesize these compounds, and as our first approach, we describe the formal synthesis of ferruginol methyl ether. Starting from tiglic aldehyde (6) and 2-bromo-5-methoxybenzoic acid (11), we synthesized the triene (3) in nine steps. The key transformation involved an acid-catalysed intramolecular Diels-Alder (IMDA) cyclisation of triene (3), leading to the formation of 12-methoxy-19-norpodocarpa-4(18),8,11,13-tetraene (14) as the primary product, with an overall yield of 17.3 % across ten steps. While the yield is modest, this approach provides a novel pathway for synthesizing abietane-type compounds. We are actively exploring strategies to improve the overall yield and developing an asymmetric synthesis variant.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


