无催化剂条件下喹喔啉-2(1H)-酮与环丁酮肟酯的光化学直接 C3 氰烷基化反应
IF 1.5
4区 化学
Q3 CHEMISTRY, ORGANIC
引用次数: 0
摘要
利用环丁酮肟酯作为氰烷基前体,无需光催化剂,首次实现了喹喔啉-2(1H)-酮的光化学直接 C3 氰烷基化反应。该方法条件温和,对环境友好,是合成 C3 氰烷基喹喔啉-2(1H)-酮的重要替代方法。本文章由计算机程序翻译,如有差异,请以英文原文为准。
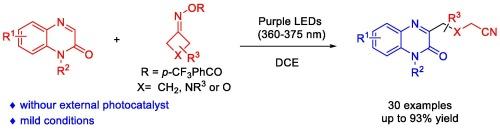
Photochemical direct C3 cyanoalkylation of quinoxalin-2(1H)-ones with cyclobutanone oxime esters under catalyst-free conditions
A photochemical direct C3 cyanoalkylation of quinoxalin-2(1H)-ones was achieved for the first time, using cyclobutanone oxime esters as cyanoalkyl precursors, without the need for a photocatalyst. The mild and environmentally friendly conditions make this protocol a valuable alternative for synthesizing C3 cyanoalkylated quinoxalin-2(1H)-ones.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Tetrahedron Letters
化学-有机化学
CiteScore
3.50
自引率
5.60%
发文量
521
审稿时长
28 days
期刊介绍:
Tetrahedron Letters provides maximum dissemination of outstanding developments in organic chemistry. The journal is published weekly and covers developments in techniques, structures, methods and conclusions in experimental and theoretical organic chemistry. Rapid publication of timely and significant research results enables researchers from all over the world to transmit quickly their new contributions to large, international audiences.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


