壳寡糖对小鼠结肠炎相关癌症的抗增殖作用:miRNA-155/TLR4/Reg3g通路的可能参与
IF 3.8
2区 农林科学
Q2 FOOD SCIENCE & TECHNOLOGY
引用次数: 0
摘要
慢性结肠炎可能会导致细胞增殖,从而带来结肠炎相关癌(CAC)的风险,CAC是结直肠癌的一种侵袭性亚型。本研究旨在探讨壳寡糖(COS)对偶氮甲烷(AOM)/葡聚糖硫酸钠(DSS)诱导的小鼠 CAC 发病的膳食干预效果和机制。500 毫克/千克剂量的壳寡糖能显著抑制 CAC 小鼠结肠中肿瘤坏死因子(TNF)-α、白细胞介素(IL)-1β 和 IL-6 的水平。与 CAC 模型对照组相比,经 COS 处理的 CAC 小鼠结肠上皮细胞表达 Ki-67 的数量和结肠细胞周期蛋白 D1 的表达水平均有所下降。服用 COS 能明显下调微(mi)RNA-155、toll 样受体(TLR)4、核因子-kappaB(NF-κB)、磷酸化信号转导和激活转录蛋白 3(pSTAT3)以及再生胰岛衍生 3 gamma(Reg3g)的表达,而上调细胞因子信号转导抑制因子 1(SOCS1)在 CAC 结肠中的表达。总之,COS对AOM/DSS诱导的结肠炎相关癌变具有保护活性,其机制可能与通过调节miRNA-155/TLR4/Reg3g途径产生的抗增殖效应有关。本文章由计算机程序翻译,如有差异,请以英文原文为准。
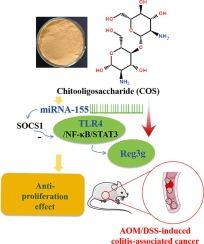
Anti-proliferation effect of chitooligosaccharide on colitis-associated cancer in mice: Possible involvement of miRNA-155/TLR4/Reg3g pathway
Chronic colonic inflammation might result in increased cell proliferation that poses risk of colitis-associated cancer(CAC), an aggressive subtype of colorectal cancer. This study aimed to investigate dietary intervention effect and mechanism of chitooligosaccharide(COS) on azoxymethane(AOM)/dextran sulfate sodium(DSS)-induced CAC development in mice. COS at dose of 500 mg/kg markedly suppressed colonic levels of tumor necrosis factor(TNF)-α, interleukin(IL)-1β, and IL-6 in CAC mice. Compared to CAC model controls, the number of colonic epithelial cells expressing Ki-67 and the colonic expression levels of cyclin D1 were decreased in COS-treated CAC mice. COS administration significantly down-regulated expression of micro(mi)RNA-155, toll-like receptor(TLR)4, nuclear factor-kappaB(NF-κB), phosphorylated signal transducer and activator of transcription protein 3(pSTAT3), and regenerating islet derived 3 gamma(Reg3g), whereas up-regulated the suppressors of cytokine signaling 1 (SOCS1) expression in CAC colons. Overall, COS exerted protective activity against AOM/DSS-induced colitis-associated carcinogenesis, mechanism of which was associated with its anti-proliferation effect possible via regulating miRNA-155/TLR4/Reg3g pathway.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Journal of Functional Foods
FOOD SCIENCE & TECHNOLOGY-
CiteScore
9.60
自引率
1.80%
发文量
428
审稿时长
76 days
期刊介绍:
Journal of Functional Foods continues with the same aims and scope, editorial team, submission system and rigorous peer review. We give authors the possibility to publish their top-quality papers in a well-established leading journal in the food and nutrition fields. The Journal will keep its rigorous criteria to screen high impact research addressing relevant scientific topics and performed by sound methodologies.
The Journal of Functional Foods aims to bring together the results of fundamental and applied research into healthy foods and biologically active food ingredients.
The Journal is centered in the specific area at the boundaries among food technology, nutrition and health welcoming papers having a good interdisciplinary approach. The Journal will cover the fields of plant bioactives; dietary fibre, probiotics; functional lipids; bioactive peptides; vitamins, minerals and botanicals and other dietary supplements. Nutritional and technological aspects related to the development of functional foods and beverages are of core interest to the journal. Experimental works dealing with food digestion, bioavailability of food bioactives and on the mechanisms by which foods and their components are able to modulate physiological parameters connected with disease prevention are of particular interest as well as those dealing with personalized nutrition and nutritional needs in pathological subjects.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


