一锅法合成羧酸功能化立方介孔二氧化硅 SBA16,用于高效去除含 Cu2+ 的废水:吸附等温线、动力学和热力学
IF 4.8
3区 材料科学
Q1 CHEMISTRY, APPLIED
引用次数: 0
摘要
含有重金属离子(如 Cu2+)的废水排放到环境中,会对自然和人类的生存健康造成无法弥补的损害。因此,从水中去除这些有毒离子绝对至关重要。本文利用氰基作为功能试剂,制备了羧基功能化 SBA16,用于吸附水中的 Cu2+。本文采用 2-氰乙基三乙氧基硅烷作为官能化试剂,通过一锅法制备了羧基官能化 SBA16。利用 WXRD、TEM、FT-IR 和 XPS 等多种技术对制备的材料进行了表征。结果表明,官能化试剂的引入并没有破坏 SBA16 原有的笼状立方介孔结构(Im3m 对称)。监测了影响 Cu2+ 去除的关键吸附因素,即 pH 值、吸附剂用量、Cu2+ 初始浓度和接触时间,并确定了最佳吸附条件。对实验数据进行了等温线和动力学研究,并采用非线性拟合方法获得了等温线和动力学参数。在 pH = 3 条件下,60 分钟内吸附量达到最大值 181.3 mg g-1。吸附动力学和吸附等温线分别遵循伪二阶(R2 = 0.999)和朗缪尔(R2 = 0.999)模型。这表明吸附过程主要涉及化学吸附和单层覆盖。热力学参数显示了吸附过程的自发和内热性质。经过 5 次循环后,该吸附剂对 Cu2+ 仍保持良好的吸附能力。这表明该吸附剂在处理含 Cu2+ 废水方面具有广阔的应用前景。本文章由计算机程序翻译,如有差异,请以英文原文为准。
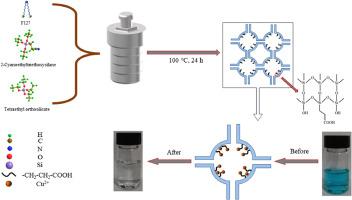
Synthesis of cubic mesoporous silica SBA16 functionalized with carboxylic acid in a one-pot process for efficient removal of wastewater containing Cu2+: Adsorption isotherms, kinetics, and thermodynamics
Wastewater containing heavy metal ions, such as Cu2+, that are released into the environment will cause irreparable damage to the nature and human health of living. It is, therefore, absolutely crucial to remove these toxic ions from water. Herein, this paper utilizes the cyano group as a functional reagent to prepare carboxyl-functionalized SBA16 for adsorbing Cu2+ in water. In this paper, 2-cyanoethyltriethoxysilane is employed as a functionalizing reagent to prepare carboxyl-functionalized SBA16 through a one-pot method. The prepared material was characterized using various techniques, such as WXRD, TEM, FT-IR, and XPS. The results indicated that the introduction of the functionalizing reagent did not disrupt the original cage-like cubic mesoporous structure (Im3m symmetry) of SBA16. Crucial adsorption factors, namely pH, adsorbent dosage, Cu2+ initial concentration, and contact time, affecting the removal of Cu2+ were monitored and the optimum adsorption conditions were determined. Isotherm and kinetic investigations were conducted and a non-linear fitting method of experimental data was used to obtain isotherm and kinetic parameters. Maximum adsorption capacity reaches 181.3 mg g−1 was achieved in 60 min at pH = 3. Adsorption kinetics and adsorption isotherm followed the pseudo-second-order (R2 = 0.999) and Langmuir (R2 = 0.999) models, respectively. This suggests that the adsorption process primarily involves chemisorption and monolayer coverage. Thermodynamic parameters showed the spontaneous and endothermic nature of the adsorption process. After 5 cycles, the adsorbent still maintains a good adsorption capacity for Cu2+. This suggests promising applications for the adsorbent in treating wastewater containing Cu2+.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Microporous and Mesoporous Materials
化学-材料科学:综合
CiteScore
10.70
自引率
5.80%
发文量
649
审稿时长
26 days
期刊介绍:
Microporous and Mesoporous Materials covers novel and significant aspects of porous solids classified as either microporous (pore size up to 2 nm) or mesoporous (pore size 2 to 50 nm). The porosity should have a specific impact on the material properties or application. Typical examples are zeolites and zeolite-like materials, pillared materials, clathrasils and clathrates, carbon molecular sieves, ordered mesoporous materials, organic/inorganic porous hybrid materials, or porous metal oxides. Both natural and synthetic porous materials are within the scope of the journal.
Topics which are particularly of interest include:
All aspects of natural microporous and mesoporous solids
The synthesis of crystalline or amorphous porous materials
The physico-chemical characterization of microporous and mesoporous solids, especially spectroscopic and microscopic
The modification of microporous and mesoporous solids, for example by ion exchange or solid-state reactions
All topics related to diffusion of mobile species in the pores of microporous and mesoporous materials
Adsorption (and other separation techniques) using microporous or mesoporous adsorbents
Catalysis by microporous and mesoporous materials
Host/guest interactions
Theoretical chemistry and modelling of host/guest interactions
All topics related to the application of microporous and mesoporous materials in industrial catalysis, separation technology, environmental protection, electrochemistry, membranes, sensors, optical devices, etc.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


