LdAMPKα2 基因敲除加速了斑蛉幼虫的生长,但抑制了几丁质的生物合成
IF 4.2
1区 农林科学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
摘要
AMPK(AMP-活化蛋白激酶)是所有真核生物物种中至关重要的细胞能量传感器。在哺乳动物中,AMPK 在维持能量平衡、调节细胞新陈代谢过程中的多重作用已被广泛研究。相比之下,AMPK 在昆虫中的功能却鲜有报道。在这里,我们成功地从一种林业鳞翅目害虫莱曼特里亚(L. dispar)身上鉴定出了三个 AMPK 亚基。在此基础上,我们特别应用 RNAi- 介导的 LdAMPKα2 基因敲除技术研究了 AMPK 信号在调节幼虫发育和几丁质生物合成中的作用。结果表明,敲除 LdAMPKα2 能显著增加悬钩子幼虫的体重,并显著上调 mTOR(哺乳动物雷帕霉素靶标)信号通路的关键基因 LdmTOR、LdS6K 和 LdSREBP1 的表达。同时,它还大大降低了 mTOR 通路的关键抑制因子 Ld4EBP 的表达。此外,沉默 LdAMPKα2 后,L. dispar 的葡萄糖水平升高,三卤糖水平降低。此外,我们还发现敲除 LdAMPKα2 后,表皮中几丁质的含量以及几丁质生物合成途径中的四个关键基因 LdGFAT、LdPAGM、LdUAP 和 LdCHSA 的表达量均显著下降。综上所述,这些结果表明,AMPK 信号在调控悬钩子幼虫的生长发育、碳水化合物代谢和几丁质生物合成中起着关键作用。这些发现拓展了我们对 AMPK 信号在昆虫体内综合调控作用的认识。本文章由计算机程序翻译,如有差异,请以英文原文为准。
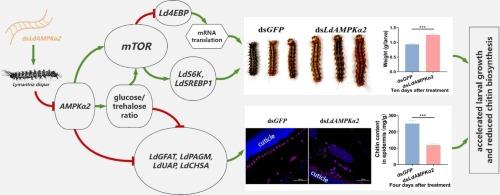
LdAMPKα2 knockdown accelerated the growth but depressed the chitin biosynthesis in Lymantria dispar larvae
AMPK (AMP-activated protein kinase) is a crucial cellular energy sensor across all eukaryotic species. Its multiple roles in maintaining energy homeostasis, regulating cellular metabolic processes have been widely investigated in mammals. In contrast, the function of AMPK in insects has been less reported. Here, we successfully identified three AMPK subunits from Lymantria dispar (L. dispar), a Lepidoptera pest in forestry. Based on that, in particular, the role of AMPK signaling in regulating larval development, as well as chitin biosynthesis was investigated by the application of RNAi-mediated LdAMPKα2 knockdown. The results indicated that knockdown of LdAMPKα2 significantly increased the body weight of L. dispar larvae, and dramatically upregulated the expression of LdmTOR, LdS6K and LdSREBP1, the key genes in mTOR (mammalian target of rapamycin) signaling pathway. While, it significantly reduced the expression of Ld4EBP, a critical repressor of mTOR pathway. Besides, the glucose level was increased and trehalose level was decreased in L. dispar after LdAMPKα2 silencing. Furthermore, we found that the chitin content in the epidermis, as well as the expressions of four key genes in the chitin biosynthesis pathway, LdGFAT, LdPAGM, LdUAP and LdCHSA, were significantly decreased after LdAMPKα2 knockdown. Taken together, these results revealed that AMPK signaling played a pivotal role in regulating the growth and development, as well as carbohydrate metabolism and chitin biosynthesis in L. dispar larvae. The findings expand our understanding of the comprehensive regulatory role of AMPK signaling in insects.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.00
自引率
8.50%
发文量
238
审稿时长
4.2 months
期刊介绍:
Pesticide Biochemistry and Physiology publishes original scientific articles pertaining to the mode of action of plant protection agents such as insecticides, fungicides, herbicides, and similar compounds, including nonlethal pest control agents, biosynthesis of pheromones, hormones, and plant resistance agents. Manuscripts may include a biochemical, physiological, or molecular study for an understanding of comparative toxicology or selective toxicity of both target and nontarget organisms. Particular interest will be given to studies on the molecular biology of pest control, toxicology, and pesticide resistance.
Research Areas Emphasized Include the Biochemistry and Physiology of:
• Comparative toxicity
• Mode of action
• Pathophysiology
• Plant growth regulators
• Resistance
• Other effects of pesticides on both parasites and hosts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


