对 Novosorb® 生物可降解增时基质治疗复杂伤口的系统性研究
Q3 Medicine
Burns open : an international open access journal for burn injuries
Pub Date : 2024-11-07
DOI:10.1016/j.burnso.2024.100378
引用次数: 0
摘要
背景在大面积深度创伤(如严重烧伤造成的创伤)后恢复真皮功能是一项重大的重建挑战。虽然分层厚皮移植(STSG)和全厚皮移植(FTSG)可以提供足够的覆盖,但真皮模板在恢复功能方面的作用正变得越来越重要。本系统综述旨在评估采用 NovoSorb® 生物可降解增殖基质 (BTM) 治疗复杂伤口的疗效、优势和局限性。截至 2024 年 4 月的所有研究均涉及使用 NovoSorb® BTM 治疗的患者。评估结果包括感染、不良事件和 BTM 损失。纳入的研究有 880 人参与,主要涉及烧伤的治疗,但也涉及其他难治性伤口。感染率为 10%,但只有极少数人报告因感染而失去 BTM。不良事件的发生率很低,大多数试验都没有报告与 BTM 相关的不良事件。结论我们的系统综述主要集中在病例系列和病例报告上,这些报告都证明了 Novosorp BTM 的疗效和罕见的副作用。然而,对比研究却很少。要全面分析使用 Novosorb (BTM) 的疗效、局限性和弊端,还需要进行更多的研究。本文章由计算机程序翻译,如有差异,请以英文原文为准。
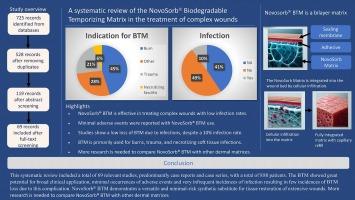
A systematic review of the Novosorb® Biodegradable Temporizing Matrix in the treatment of complex wounds
Background
Restoring a functional dermis following extensive, deep wounds, such as those caused by severe burns, presents a significant reconstructive challenge. Although split thickness skin graft (STSG) and full thickness skin graft (FTSG) can provide adequate coverage, dermal templates are becoming increasingly important in restoring function. The purpose of this systematic review is to assess the efficacy, advantages and limitations of employing NovoSorb® Biodegradable Temporizing Matrix (BTM) in the treatment of complex wounds.
Methods
The systematic review was carried out in accordance with PRISMA and MOOSE guidelines when appropriate. All studies until April 2024 involving patients treated with NovoSorb® BTM were considered. Infection, adverse events, and BTM loss were among the outcomes evaluated.
Results
We identified 725 studies, and 69 were included after screening. The included studies involved 880 participants and were mostly concerned with the management of burns, but other difficult wounds were also addressed. The infection rate was 10%, yet only few reported losing their BTM as a result of this consequence. The incidence of adverse events was low, with the majority of trials reporting no adverse events related to BTM.
Conclusion
Our systematic review focused primarily on case series and case reports that demonstrated the efficacy of Novosorp BTM and the rarity of side effects. However, there were very few comparison research. More research is needed to fully analyze the efficacy, limitations, and downsides of using Novosorb (BTM).
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
1.20
自引率
0.00%
发文量
0
审稿时长
15 weeks

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


