可定制且成本低廉的三维打印透孔装置与三维细胞培养相结合,用于渗透性检测
IF 2
Q3 ENGINEERING, ELECTRICAL & ELECTRONIC
引用次数: 0
摘要
基于渗透性的检测通常用于评估肠道屏障功能,它依赖于使用一个 transwell 插入物作为基本隔室。该装置由附着在插入物底部的半透膜组成,将系统分成顶端区和基底侧区。然而,商业化的插入物根据不同的应用有不同的孔径,只能提供二维细胞培养的平面。在此,我们介绍了一种简单、低成本的三维打印 transwell 装置,以及一种将插入物功能化用于纸质三维细胞培养的可靠方法。这种三维打印装置由聚乳酸(PLA)长丝制成,纸膜用于支持 HT-29 细胞进行肠道渗透性评估。在培养 HT-29 细胞 48 小时和 72 小时后,该装置显示出良好的生物相容性,细胞存活率分别达到 97% 和 98%。结合荧光图像,细胞直接附着在 Matrigel 功能化纸的微纤维网络上,这表明功能化纸具有生物相容性和生物活性。此外,在更合适的培养微环境中,扫描电子显微镜(SEM)分析显示细胞特征分化为分泌粘液的细胞,细胞表面微绒毛的形成就是证明,绒毛蛋白-1 的免疫荧光染色进一步证实了这一点。为了证明三维打印 transwell 装置的可用性,使用化学和生物刺激处理方法评估了肠道通透性。使用 FITC 葡聚糖的渗透性结果验证了不同水平的相对荧光强度单位(RFU)与 CellTrackerTM 活细胞橙色之间的联系。因此,这种三维打印的 transwell 装置提供了一种直接、经济高效的方法,可在许多实验室环境中制造定制装置,使其成为市场上无法定制的 transwell 装置的可行替代品。本文章由计算机程序翻译,如有差异,请以英文原文为准。
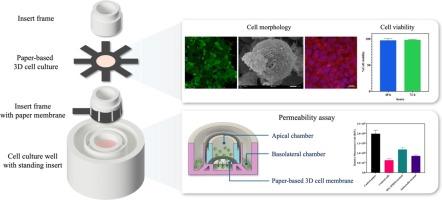
A customizable and low-cost 3D-printed transwell device coupled with 3D cell culture for permeability assay
The permeability-based assay is commonly used to assess intestinal barrier function, and it relies on using a transwell insert as an essential compartment. The device consists of a semipermeable membrane that is attached at the bottom of the insert and splits the system into the apical and basolateral compartments. However, commercial inserts are standardized with different pore sizes based on the application and offer only a flat plane of two-dimensional cell culture. Herein, we present a simple, low-cost 3D-printed transwell device and a robust method to functionalize the inserts for paper-based 3D cell culture. This 3D-printed device was fabricated from a polylactic acid (PLA) filament, and a paper membrane used to support HT-29 cells for intestinal permeability assessment. A device showed good biocompatibility when culturing HT-29 cells for 48 and 72 h with 97 % and 98 % cell viability, respectively. Together with fluorescence images, cells were attached directly to the microfiber networks of a Matrigel-functionalized paper, indicating that the functionalized paper is biocompatible and bioactive. Furthermore, in a more appropriate culture microenvironment, SEM analyses revealed cellular features differentiating into mucus-secreting cells, evidenced by the formation of microvilli on the cell surface, which was further confirmed by immunofluorescence staining of villin-1. To demonstrate the usability of the 3D-printed transwell device, intestinal permeability was assessed using both chemical and biological stimulation treatments. The permeability results employing FITC-dextran validated the association between a different level of relative fluorescence intensity unit (RFU) and the orange color of live cells by CellTrackerTM. As a result, this 3D-printed transwell device provides a straightforward and cost-effective method for manufacturing a device for customization in many laboratory settings, making it a feasible alternative to marketed transwell devices that do not allow for customization.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

HardwareX
Engineering-Industrial and Manufacturing Engineering
CiteScore
4.10
自引率
18.20%
发文量
124
审稿时长
24 weeks
期刊介绍:
HardwareX is an open access journal established to promote free and open source designing, building and customizing of scientific infrastructure (hardware). HardwareX aims to recognize researchers for the time and effort in developing scientific infrastructure while providing end-users with sufficient information to replicate and validate the advances presented. HardwareX is open to input from all scientific, technological and medical disciplines. Scientific infrastructure will be interpreted in the broadest sense. Including hardware modifications to existing infrastructure, sensors and tools that perform measurements and other functions outside of the traditional lab setting (such as wearables, air/water quality sensors, and low cost alternatives to existing tools), and the creation of wholly new tools for either standard or novel laboratory tasks. Authors are encouraged to submit hardware developments that address all aspects of science, not only the final measurement, for example, enhancements in sample preparation and handling, user safety, and quality control. The use of distributed digital manufacturing strategies (e.g. 3-D printing) is encouraged. All designs must be submitted under an open hardware license.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


