了解掺镁钛酸铜铋(BCTO)在氧进化反应中的电催化作用
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
由于水氧化对可再生能源的生产和储存具有重要意义,因此设计高效的水氧化电催化剂在催化领域变得越来越重要。从水中制取氢气(H2)的过程因水分裂过程的动力学非常缓慢而受到阻碍。要了解氧进化反应的主要障碍,还需要增强有效的氧进化反应(OER)电催化剂。本文研究了掺镁钛酸铋铜(Mg-BCTO)作为水电解 OER 的高效催化剂的电化学活性,水电解是可持续能源制氢的关键步骤。利用 XRD、FT-IR、拉曼、扫描电镜、TEM、XPS、CV、EIS 和 Tafel 极化分析,对合成材料(包括不同化学计量的掺镁 BCTO)进行了全面的物理和电化学表征。令人瞩目的是,掺杂了 Mg0.1 的 BCTO 表现出卓越的性能,在 265 mV 的超低过电位 (η10) 和 92 mV dec-1 的塔菲尔斜率条件下,其电流密度达到了 10 mA cm-2。这一发现不仅彰显了掺镁 BCTO 的电催化效率,还将其定位为开发高活性、高稳定性水氧化催化剂的理想模型,有助于推动清洁能源技术的发展。本文章由计算机程序翻译,如有差异,请以英文原文为准。
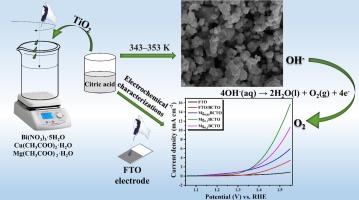
Understanding the electrocatalytic role of magnesium doped bismuth copper titanate (BCTO) in oxygen evolution reaction
Designing high-efficiency electrocatalysts for water oxidation has become increasingly important in the catalysis field owing to its implications for renewable energy production and storage. The production of hydrogen (H2) from water is hampered by the very sluggish kinetics of the water-splitting process. Enhancement of effective oxygen evolution reaction (OER) electrocatalysts is also required to understand the primary barrier to OER. This article investigates the electrochemical activity of magnesium-doped bismuth copper titanate (Mg-BCTO) as an efficient catalyst for the OER in water electrolysis, a critical step in hydrogen production for sustainable energy. The synthesized materials, including various stoichiometries of Mg-doped BCTO, undergo thorough physical and electrochemical characterization using XRD, FT-IR, Raman, SEM, TEM, XPS, CV, EIS, and Tafel polarization analyses. Remarkably, Mg0.1 doped BCTO demonstrates superior performance, achieving a current density of 10 mA cm−2 at a very low overpotential (η10) of 265 mV and with a Tafel slope of 92 mV dec−1. This finding not only highlights the electrocatalytic efficiency of Mg doped BCTO but also positions it as a promising model for the development of highly active and stable water oxidizing catalysts, contributing to the advancement of clean energy technologies.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


