掺氮碳涂层 Na3V2O2(PO4)2F 作为高性能钠离子电池的阴极
IF 4.1
3区 化学
Q1 CHEMISTRY, ANALYTICAL
引用次数: 0
摘要
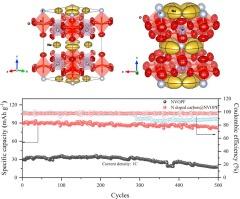
Na3V2O2(PO4)2F (NVOPF)是一种具有高电压、高能量密度和热稳定性等优点的阴极,被视为钠离子电池阴极的理想候选材料。然而,NVOPF 在储钠方面表现不佳。在此,我们报告了一种由盐酸多巴胺在高温下生成的掺氮碳的 NVOPF 复合阴极。首先,利用中子功率衍射(NPD)确定了纯 NVOPF 在不同温度下的结构,包括氧配位和 Na+ 的迁移率。掺氮碳层有利于电子传导,防止 NVOPF 纳米粒子团聚,从而在钠离子插入/萃取过程中促进电子和离子的高效转移,从而提高材料结构的稳定性。这些结果表明,掺杂 N 的碳@NVOPF 阴极材料具有出色的电化学性能(0.1C 时为 111mAh/g,1C 时为 85mAh/g),同时在 500 次循环后仍能保持 91.06% 的容量。高效的电荷转移动力学提高了电化学性能,为我们提供了新的视角,而且该方法还可有效地应用于其他阴极材料。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Nitrogen-doped carbon coated Na3V2O2(PO4)2F as a cathode for high-performance sodium-ion batteries
Na3V2O2(PO4)2F (NVOPF), a cathode known for the advantages of high voltage, exceptional energy density, and thermal stability, is seen as a promising candidate for sodium-ion battery cathodes. However, the NVOPF exhibits poor performance in sodium storage. Here, we report a NVOPF composite cathode with a nitrogen-doped carbon produced from dopamine hydrochloride at an elevated temperature. Firstly, neutron power diffraction (NPD) is employed to determine the structure of pure NVOPF at different temperatures, including oxygen coordination and the mobility of Na+. The nitrogen-doped carbon layer facilitates electron conduction, prevents NVOPF nanoparticle agglomeration, and thus promotes efficient electron and ion transfer during sodium ion insertion/extraction process, thereby improving the stability of the material structure. These results indicate that the N doped carbon@NVOPF cathode material exhibits outstanding electrochemical performance (111mAh/g at 0.1C and 85mAh/g at 1C), while maintaining a capacity retention of 91.06 % after 500 cycles. The enhanced electrochemical performance attributes to efficient charge transfer kinetics offer novel perspectives, and the method can be effectively adapted to other cathode materials.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊
CiteScore
7.80
自引率
6.70%
发文量
912
审稿时长
2.4 months
期刊介绍:
The Journal of Electroanalytical Chemistry is the foremost international journal devoted to the interdisciplinary subject of electrochemistry in all its aspects, theoretical as well as applied.
Electrochemistry is a wide ranging area that is in a state of continuous evolution. Rather than compiling a long list of topics covered by the Journal, the editors would like to draw particular attention to the key issues of novelty, topicality and quality. Papers should present new and interesting electrochemical science in a way that is accessible to the reader. The presentation and discussion should be at a level that is consistent with the international status of the Journal. Reports describing the application of well-established techniques to problems that are essentially technical will not be accepted. Similarly, papers that report observations but fail to provide adequate interpretation will be rejected by the Editors. Papers dealing with technical electrochemistry should be submitted to other specialist journals unless the authors can show that their work provides substantially new insights into electrochemical processes.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


