苦味受体 T2R14-Gαi 偶联介导囊性纤维化患者对微生物法定量传感分子的先天性免疫反应
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
囊性纤维化(CF)是一种常染色体隐性遗传病,以微生物感染和肺功能进行性下降为特征,导致严重的发病率和死亡率。苦味受体 T2R14 是一种化学感觉受体,在气道中显著表达。通过细胞检测和 T2R14 在 CF 和非 CF 患者支气管上皮细胞中的敲除相结合的方法,我们观察到 T2R14 在检测细菌和真菌信号以及增强宿主先天性免疫反应中起着至关重要的作用。Gαi蛋白在CF支气管上皮细胞中的表达增强,T2R14-Gαi特异性信号转导导致钙动员增加。敲除 T2R14 会导致缺乏法定量感应的细菌菌株对先天性免疫的激活降低。研究结果表明,T2R14有助于抵御微生物感染,因此可能在CF气道上皮细胞的先天性免疫防御中发挥重要作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
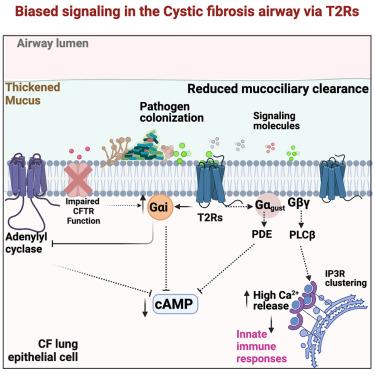
Bitter taste receptor T2R14-Gαi coupling mediates innate immune responses to microbial quorum sensing molecules in cystic fibrosis
Cystic fibrosis (CF) is an autosomal recessive disease characterized by microbial infection and progressive decline in lung function, leading to significant morbidity and mortality. The bitter taste receptor T2R14 is a chemosensory receptor that is significantly expressed in airways. Using a combination of cell-based assays and T2R14 knockdown in bronchial epithelial cells from CF and non-CF individuals, we observed that T2R14 plays a crucial role in the detection of bacterial and fungal signals and enhances host innate immune responses. Expression of Gαi protein is enhanced in CF bronchial epithelial cells and T2R14-Gαi specific signaling leads to increased calcium mobilization. Knockdown of T2R14 leads to reduced innate immune activation by bacterial strains deficient in quorum sensing. The results demonstrate that T2R14 helps protect against microbial infection and thus may play an important role in the innate immune defense of the CF airway epithelium.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


