维生素 D 通过抑制 DNMT1 来影响 TGFβ1/Smad3 通路,从而减轻高密度脂蛋白胆固醇诱导的肝纤维化
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
越来越多的证据表明,维生素 D(VD)具有脂肪代谢和免疫相关特性,可通过影响 DNA 甲基化来预防相关代谢性疾病,但结果并不一致。同时,维生素 D 对代谢相关性脂肪肝(MAFLD)进展的相对精确的分子代谢作用仍不确定。在此,我们报告了补充 VD 在缓解高脂饮食(HFD)诱导的 MAFLD 方面前所未有的作用和可能机制。我们的研究结果表明,随着时间的推移,高脂饮食诱导的 MAFLD 代谢紊乱会加剧,并具有一定的时间反应依赖性,同时伴有 VD 代谢物的减少。在体内和体外补充足够的 VD 后,所有这些问题都可以得到缓解。这部分是通过抑制 DNMT1 的表达,逆转 VD 代谢基因和 TGFβR1 的表观遗传模式,最终触发 TGFβ1/Smad3 通路,导致 MAFLD 的发生。此外,通过基因沉默 DNMT1 的处理削弱了 VD 的保护作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
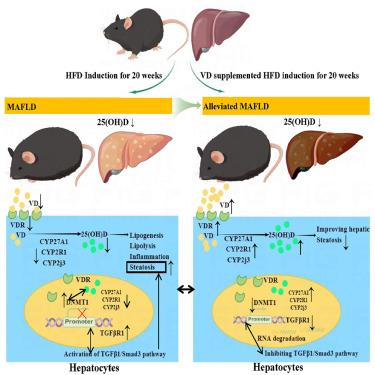
Vitamin D alleviates HFD-induced hepatic fibrosis by inhibiting DNMT1 to affect the TGFβ1/Smad3 pathway
Increasing evidence points toward vitamin D (VD) having lipometabolism and immune-related properties to protect against related metabolic diseases through influencing DNA methylation with inconsistent results. Simultaneously, its relatively precise molecular metabolism on the progression of metabolic-associated fatty liver disease (MAFLD) remains uncertain. Here, we report an unprecedented role and possible mechanism for VD supplementation on the alleviation of high-fat diet (HFD)-induced MAFLD. Over time, our results demonstrated that metabolic disorders in the HFD-induced MAFLD were aggravated with a certain time-response dependence and accompanied by reduced VD metabolites. All these could be alleviated under sufficient VD supplementation in vivo and vitro. It was partially by inhibiting the expressions of DNMT1 to reverse the epigenetic patterns on the VD metabolism genes and TGFβR1, which ultimately triggered the TGFβ1/Smad3 pathway to result in the development of MAFLD. Furthermore, the protective effects of VD were weakened by the treatment with gene silencing of DNMT1.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


