用于先进储氢应用的钛基 X2TiH5(X = Mg、Ca、Sr)氢化物的第一性原理研究
IF 2
3区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
摘要
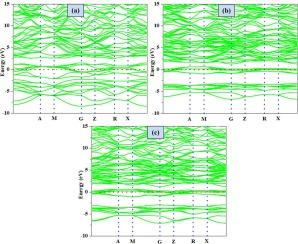
解决移动应用中氢气存储这一紧迫问题的潜在解决方案在于固体材料的潜在贡献。此外,人们还对过氧化物氢化物材料进行了广泛研究,以提高其在储氢领域的效率。本研究的重点是对 X2TiH5(X = Mg、Ca 和 Sr)包晶型氢化物进行计算研究,同时考虑到其不同的物理性质及其在储氢中的潜在用途。通过分析 X2TiH5(X = Mg、Ca 和 Sr)的负形成平衡,评估了它们的热力学稳定性。Sr2TiH5 呈四方结构的化合物具有最高的计算晶格常数,即 a = b = 5.69 Å 和 c = 7.91 Å。此外,金属氢化物有望成为储氢应用的潜在竞争者。此外,还对所有化合物的光学特性进行了量化。最后,计算得出 Mg2TiH5 的储氢能力为 4.97 wt%,Ca2TiH5 为 3.78 wt%,Sr2TiH5 为 2.21 wt%。这项研究表明 X2TiH5(X = Mg、Ca 和 Sr)具有储氢潜力,尽管 Mg2TiH5 和 Ca2TiH5 是唯一符合美国能源部 2020 年标准的化合物。本文章由计算机程序翻译,如有差异,请以英文原文为准。
First-Principles study of Ti-Based X2TiH5 (X = Mg, Ca, Sr) hydrides for Advanced hydrogen storage applications
The potential solution to the urgent issue of hydrogen storage in mobile applications is in the potential contribution of solid materials. Moreover, extensive study has been carried out on perovskite hydride materials to improve their efficiency in the field of hydrogen storage. The present work focuses on the computational investigation of X2TiH5 (X = Mg, Ca, and Sr) perovskite-type hydrides, taking into account diverse physical properties and their potential uses in hydrogen storage. The thermodynamic stability of X2TiH5 (X = Mg, Ca, and Sr) was assessed by analyzing their negative formation equilibrium. The compounds exhibiting tetragonal structures with Sr2TiH5 have the highest computed lattice constants, namely a = b = 5.69 Å and c = 7.91 Å. The electrical properties unequivocally indicate that the molecules being investigated have a metallic nature. Furthermore, the metallic hydrides provide promising possibilities as potential contenders for hydrogen storage applications. Furthermore, the optical properties of all the compounds were quantified. Finally, the calculated hydrogen storage capacities are 4.97 wt% for Mg2TiH5, 3.78 wt% for Ca2TiH5 and 2.21 wt% for Sr2TiH5. This study demonstrated X2TiH5 (X = Mg, Ca and Sr) have the potential for hydrogen storage, although Mg2TiH5 and Ca2TiH5 are the only compounds that meets the US-DOE criterion for 2020.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

Chemical Physics
化学-物理:原子、分子和化学物理
CiteScore
4.60
自引率
4.30%
发文量
278
审稿时长
39 days
期刊介绍:
Chemical Physics publishes experimental and theoretical papers on all aspects of chemical physics. In this journal, experiments are related to theory, and in turn theoretical papers are related to present or future experiments. Subjects covered include: spectroscopy and molecular structure, interacting systems, relaxation phenomena, biological systems, materials, fundamental problems in molecular reactivity, molecular quantum theory and statistical mechanics. Computational chemistry studies of routine character are not appropriate for this journal.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


