肠道微生物群通过调节先天免疫力增强蟑螂对伤寒沙门氏菌的抵抗力
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
蟑螂对秋伤寒沙门氏菌(S. Typhimurium)感染的敏感性在种群内和种群间存在无法解释的差异。在这里,我们发现肠道微生物群对蟑螂摄入的鼠伤寒沙门氏菌的定植具有保护作用。我们进一步研究了这种效应的两种潜在机制,结果表明肠道中的共生细菌在蟑螂粪便中生长期间不会与鼠伤寒杆菌竞争,而是会促进宿主抗菌肽基因的表达,从而抑制鼠伤寒杆菌的感染。最后,我们确定微生物群的绝对丰度或总体多样性都与感染易感性无关。相反,我们发现了几个表现出个体间丰度差异的少数细菌类群,它们是遗传相似个体感染易感性的关键指标。这些发现揭示了蟑螂作为无脊椎动物种间微生物相互作用模型的潜力,并提供了对病媒传播沙门氏菌的深入了解,表明蟑螂的微生物群可以作为减少病原体传播的目标。本文章由计算机程序翻译,如有差异,请以英文原文为准。
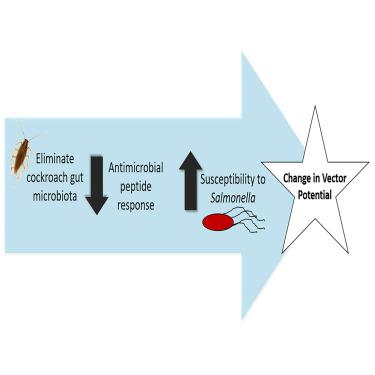
The gut microbiota confers resistance against Salmonella Typhimurium in cockroaches by modulating innate immunity
Cockroaches exhibit unexplained intra- and interpopulation variation in susceptibility to Salmonella enterica serovar Typhimurium (S. Typhimurium) infection. Here, we show that the gut microbiota has a protective effect against colonization by ingested S. Typhimurium in cockroaches. We further examine two potential mechanisms for this effect, showing that commensal bacteria present in the gut do not compete with S. Typhimurium during growth in cockroach feces, but rather prime expression of host antimicrobial peptide genes that suppress S. Typhimurium infection. Lastly, we determine that neither absolute abundance of the microbiota nor its overall diversity is linked to infection susceptibility. Instead, we identify several minority bacterial taxa that exhibit interindividual variation in abundance as key indicators of infection susceptibility among genetically similar individuals. These findings illuminate the potential of cockroaches as an invertebrate model for interspecies microbial interactions and provide insight into vector-borne Salmonella transmission, suggesting that the microbiota of cockroaches could be targeted to reduce pathogen transmission.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


