琼脂糖水凝胶介导的电穿孔法,用于在气液界面培养视网膜组织
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
在空气-液体界面培养体外视网膜和其他组织具有优势,可实现更有效的气体交换。然而,向这些培养物传递基因可能具有挑战性。电穿孔是一种快速、稳健的基因递送方法,但通常需要浸没在液体缓冲液中才能产生电流。我们开发了一种无需浸没的电穿孔技术,它在正极和视网膜之间加入了一个琼脂糖水凝胶盘。内视网膜神经元和 Müller 胶质被转染,在培养时间延长后,Müller 胶质的转染倾向增加。我们还观察到电刺激后 Müller 胶质中的 BrdU 结合增加,以及不同启动子的表达载体在检测转染细胞时的差异。这种方法提高了我们利用体外视网膜组织进行基因研究的能力,并可用于在气液界面培养的其他组织。本文章由计算机程序翻译,如有差异,请以英文原文为准。
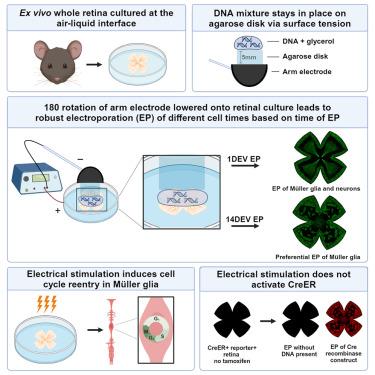
Agarose hydrogel-mediated electroporation method for retinal tissue cultured at the air-liquid interface
It is advantageous to culture the ex vivo retina and other tissues at the air-liquid interface to allow for more efficient gas exchange. However, gene delivery to these cultures can be challenging. Electroporation is a fast and robust method of gene delivery, but typically requires submergence in liquid buffer for electrical current flow. We have developed a submergence-free electroporation technique that incorporates an agarose hydrogel disk between the positive electrode and retina. Inner retinal neurons and Müller glia are transfected with increased propensity toward Müller glia transfection after extended time in culture. We also observed an increase in BrdU incorporation in Müller glia following electrical stimulation, and variation in detection of transfected cells from expression vectors with different promoters. This method advances our ability to use ex vivo retinal tissue for genetic studies and should be adaptable for other tissues cultured at an air-liquid interface.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


