DT40 细胞有丝分裂过程中 CENP-T-Mis12 复合物相互作用的分子细节和磷酸化调控
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
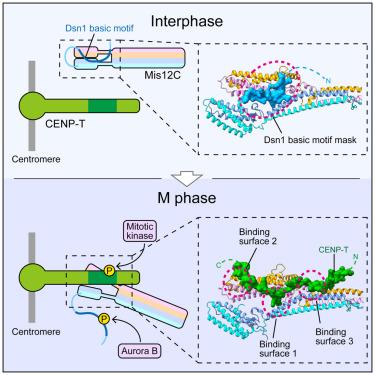
为了建立微管与姐妹染色单体的双极连接,需要在中心染色质上组装内部动点核亚复合物--构成型中心相关网络(CCAN),并招募称为 KMN 网络的微管结合亚复合物。由于CCAN蛋白CENP-C和CENP-T分别与KMN的Mis12复合体(Mis12C)结合,因此很难评估细胞中每种相互作用的意义。在这里,我们利用缺乏 CENP-C-Mis12C 相互作用的鸡 DT40 细胞展示了 CENP-T-Mis12C 相互作用的分子细节。利用 AlphaFold 预测结合细胞生物学和生化分析,我们确定了 CENP-T-Mis12C 相互作用的三个结合面,证明了每个界面对于细胞中将 Mis12C 募集到 CENP-T 非常重要。这种相互作用通过三个结合面受到 Dsn1(Mis12C 的一个组成部分)和 CENP-T 的双重磷酸化的协同调控,从而确保了 CENP-T-Mis12C 强有力的相互作用和有丝分裂的正常进行。这些发现加深了我们对细胞中动子核心组装的理解。本文章由计算机程序翻译,如有差异,请以英文原文为准。
Molecular details and phosphoregulation of the CENP-T-Mis12 complex interaction during mitosis in DT40 cells
To establish bipolar attachments of microtubules to sister chromatids, an inner kinetochore subcomplex, the constitutive centromere-associated network (CCAN), is assembled on centromeric chromatin and recruits the microtubule-binding subcomplex called the KMN network. Since CCAN proteins CENP-C and CENP-T independently bind to the Mis12 complex (Mis12C) of KMN, it is difficult to evaluate the significance of each interaction in cells. Here, we demonstrate the molecular details of the CENP-T-Mis12C interaction using chicken DT40 cells lacking the CENP-C-Mis12C interaction. Using AlphaFold predictions combined with cell biological and biochemical analyses, we identified three binding surfaces of the CENP-T-Mis12C interaction, demonstrating that each interface is important for recruiting Mis12C to CENP-T in cells. This interaction, via three interaction surfaces, is cooperatively regulated by dual phosphorylation of Dsn1 (a Mis12C component) and CENP-T, ensuring a robust CENP-T-Mis12C interaction and proper mitotic progression. These findings deepen our understanding of kinetochore assembly in cells.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


