16p11.2缺失小鼠自闭症模型中片段睡眠和记忆缺陷的回路机制
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
自闭症谱系障碍(ASD)儿童普遍存在睡眠障碍。令人震惊的是,睡眠问题与自闭症症状(如记忆障碍)的严重程度呈正相关。然而,自闭症谱系障碍儿童睡眠障碍和认知缺陷的神经机制在很大程度上尚未得到研究。在这里,我们发现在16p11.2缺失的ASD小鼠模型中,非快速眼动睡眠(NREMs)是片段的。睡眠片段化的程度反映在非快速眼动睡眠(NREMs)期间,脑室小脑去甲肾上腺素能(LC-NE)神经元活动中的钙瞬态数量增加。相反,光遗传抑制 LC-NE 神经元和使用氯尼丁药物阻断去甲肾上腺素能传导可巩固睡眠。此外,抑制 LC-NE 神经元还能恢复记忆。最后,狂犬病介导的突触前神经元筛查发现,16p11.2 缺失小鼠的 LC-NE 神经元与睡眠和记忆调节区域的连接发生了改变。我们的研究结果确定了 LC-NE 系统在 ASD 中调节睡眠稳定性和记忆的关键作用。本文章由计算机程序翻译,如有差异,请以英文原文为准。
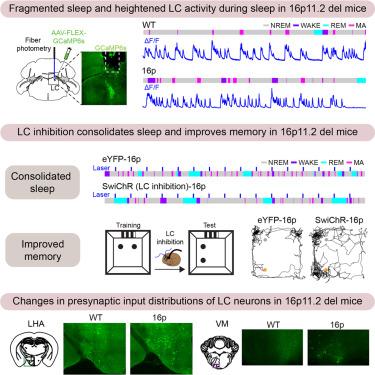
Circuit mechanism underlying fragmented sleep and memory deficits in 16p11.2 deletion mouse model of autism
Sleep disturbances are prevalent in children with autism spectrum disorder (ASD). Strikingly, sleep problems are positively correlated with the severity of ASD symptoms, such as memory impairment. However, the neural mechanisms underlying sleep disturbances and cognitive deficits in ASD are largely unexplored. Here, we show that non-rapid eye movement sleep (NREMs) is fragmented in the 16p11.2 deletion mouse model of ASD. The degree of sleep fragmentation is reflected in an increased number of calcium transients in the activity of locus coeruleus noradrenergic (LC-NE) neurons during NREMs. In contrast, optogenetic inhibition of LC-NE neurons and pharmacological blockade of noradrenergic transmission using clonidine consolidate sleep. Furthermore, inhibiting LC-NE neurons restores memory. Finally, rabies-mediated screening of presynaptic neurons reveals altered connectivity of LC-NE neurons with sleep- and memory-regulatory regions in 16p11.2 deletion mice. Our findings identify a crucial role of the LC-NE system in regulating sleep stability and memory in ASD.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


