饮食热量限制通过调节Teff/Treg平衡保护实验性自身免疫性葡萄膜炎
IF 4.6
2区 综合性期刊
Q1 MULTIDISCIPLINARY SCIENCES
引用次数: 0
摘要
葡萄膜炎是一种自身免疫性疾病,通常会导致失明。CD4+ T细胞,包括调节性T细胞(Tregs)和效应T细胞(Th1和Th17),在其发病机制中起着至关重要的作用。热量限制(CR)已被证明可以缓解自身免疫性疾病。然而,人们对热量限制对实验性自身免疫性葡萄膜炎(EAU)的影响还知之甚少。本研究使用单细胞 RNA 测序分析了小鼠在自由饮食(AL)和 CR 饮食条件下,有或没有 EAU 的颈部引流淋巴结。CR增加了Tregs,改变了免疫细胞代谢,减轻了EAU症状,并下调了炎症和糖酵解基因。流式细胞术证实了CR对Th1和Th17增殖的抑制作用以及对Treg增殖的促进作用。CR 还通过抑制 PI3K/AKT/c-Myc 通路和减少 Th17 细胞中的 GM-CSF 来平衡 CD4+ T 细胞。这些发现表明,CR 是治疗自身免疫性疾病的一种潜在策略。本文章由计算机程序翻译,如有差异,请以英文原文为准。
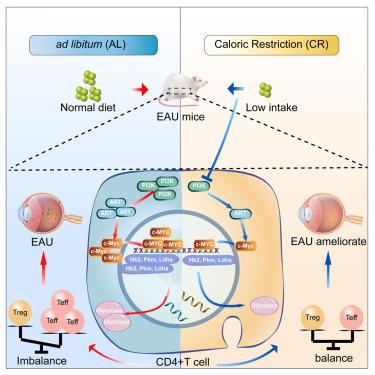
Dietary caloric restriction protects experimental autoimmune uveitis by regulating Teff/Treg balance
Uveitis, an autoimmune disease, often leads to blindness. CD4+ T cells, including regulatory T cells (Tregs) and effector T cells (Th1 and Th17), play a critical role in its pathogenesis. Caloric restriction (CR) has been shown to alleviate autoimmune diseases. However, careful characterization of the impact of CR on experimental autoimmune uveitis (EAU) is poorly understood. This study used single-cell RNA sequencing to analyze cervical draining lymph nodes in mice under ad libitum (AL) and CR diets, with or without EAU. CR increased Tregs, altered immune cell metabolism, reduced EAU symptoms, and downregulated inflammatory and glycolysis genes. Flow cytometry confirmed CR’s inhibitory effect on Th1 and Th17 proliferation and its promotion of Treg proliferation. CR also balanced CD4+ T cells by inhibiting the PI3K/AKT/c-Myc pathway and reducing GM-CSF in Th17 cells. These findings suggest CR as a potential therapeutic strategy for autoimmune diseases.
求助全文
通过发布文献求助,成功后即可免费获取论文全文。
去求助
来源期刊

iScience
Multidisciplinary-Multidisciplinary
CiteScore
7.20
自引率
1.70%
发文量
1972
审稿时长
6 weeks
期刊介绍:
Science has many big remaining questions. To address them, we will need to work collaboratively and across disciplines. The goal of iScience is to help fuel that type of interdisciplinary thinking. iScience is a new open-access journal from Cell Press that provides a platform for original research in the life, physical, and earth sciences. The primary criterion for publication in iScience is a significant contribution to a relevant field combined with robust results and underlying methodology. The advances appearing in iScience include both fundamental and applied investigations across this interdisciplinary range of topic areas. To support transparency in scientific investigation, we are happy to consider replication studies and papers that describe negative results.
We know you want your work to be published quickly and to be widely visible within your community and beyond. With the strong international reputation of Cell Press behind it, publication in iScience will help your work garner the attention and recognition it merits. Like all Cell Press journals, iScience prioritizes rapid publication. Our editorial team pays special attention to high-quality author service and to efficient, clear-cut decisions based on the information available within the manuscript. iScience taps into the expertise across Cell Press journals and selected partners to inform our editorial decisions and help publish your science in a timely and seamless way.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


